Test: Structure of the Atom- Case Based Type Questions - Class 9 MCQ
12 Questions MCQ Test Class 9: Additional Practice - Test: Structure of the Atom- Case Based Type Questions
Direction: The electronic configuration of an element ‘X’ is 2, 8, 2 :
Q. How many Valence electrons are there in element X?
Direction: The electronic configuration of an element ‘X’ is 2, 8, 2 :
Q. The number of electrons present in the atom of element ‘X’ is __________.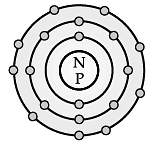

| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Direction: The electronic configuration of an element ‘X’ is 2, 8, 2 :
Q. The Valency of element X is:
Direction: The electronic configuration of an element ‘X’ is 2, 8, 2 :
Q. The element X is:
Direction: In the following table the mass number and the atomic number of certain elements are given. Study the given data and answer the following questions :
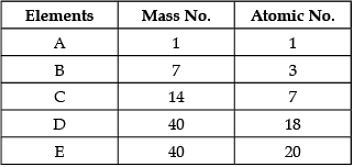
Q. The element which can form a cation:
Direction: In the following table the mass number and the atomic number of certain elements are given. Study the given data and answer the following questions :
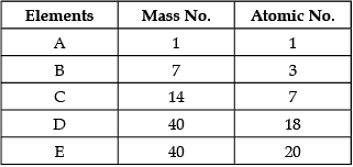
Q. Any two elements are Isobars:
Direction: In the following table the mass number and the atomic number of certain elements are given. Study the given data and answer the following questions :
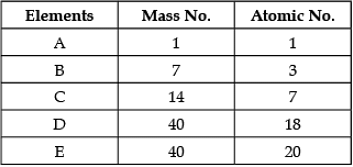
Q. Which element will form an anion?
Direction: In the following table the mass number and the atomic number of certain elements are given. Study the given data and answer the following questions :
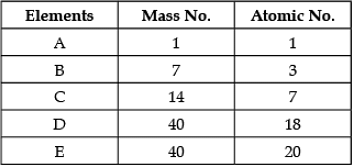
Q. Which element is a noble gas?
Direction: Read the following passage and answer the following questions.
In order to overcome the objections raised against Rutherford’s model of the atom, Neil Bohr put forward the following postulates about the model of an atom.
Q. Who amended Rutherford’s short comings?
Direction: Read the following passage and answer the following questions.
In order to overcome the objections raised against Rutherford’s model of the atom, Neil Bohr put forward the following postulates about the model of an atom.
Q. Atoms are made up of ______, _____ and ______.
Direction: Read the following passage and answer the following questions.
In order to overcome the objections raised against Rutherford’s model of the atom, Neil Bohr put forward the following postulates about the model of an atom.
Q. Atomic mass is the sum of:
Direction: Read the following passage and answer the following questions.
In order to overcome the objections raised against Rutherford’s model of the atom, Neil Bohr put forward the following postulates about the model of an atom.
Q. The number of electrons that K-shell and L-shell can accommodate:
|
4 docs|108 tests
|

















