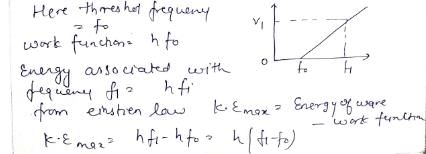Test: Dual Nature of Radiation & Matter - 3 - NEET MCQ
26 Questions MCQ Test Topic-wise MCQ Tests for NEET - Test: Dual Nature of Radiation & Matter - 3
In a photo-emissive cell, with exciting wavelength l, the maximum kinetic energy of electron is K. If the exciting wavelength is changed to 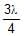 the kinetic energy of the fastest emitted electron will be :
the kinetic energy of the fastest emitted electron will be :
The frequency and the intensity of a beam of light falling on the surface of photoelectric material are increased by a factor of two (Treating efficiency of photoelectron generation as constant). This will :
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Light coming from a discharge tube filled with hydrogen falls on the cathode of the photoelectric cell. The work function of the surface of cathode is 4eV. Which one of the following values of the anode voltage (in Volts) with respect to the cathode will likely to make the photo current zero.
Let K1 be the maximum kinetic energy of photoelectrons emitted by a light of wavelength l1 and K2 corresponding to l2. If l1 = 2l2, then :
In a photoelectric experiment, the potential difference V that must be maintained between the illuminated surface and the collector so as just to prevent any electron from reaching the collector is determined for different frequencies f of the incident illumination. The graph obtained is shown. The maximum kinetic energy of the electrons emitted at frequency f1 is
In photoelectric effect, stopping potential depends on
In the experiment on photoelectric effect using light having frequency greater than the threshold frequency, the photocurrent will certainly increase when
Two electrons are moving with the same speed v. One electron enters a region of uniform electric field while the other enters a region of uniform magnetic field, then after sometime if the de-Broglie wavelengths of the two are l1 and l2 then :
An electron in hydrogen atom first jumps from second excited state to first excited state and then, from first excited state to ground state. Let the ratio of wavelength, momentum and energy of photons in the two cases by x, y and z, then select the wrong answers :
An electron is in an excited state in hydrogen-like atom. It has a total energy of –3.4 eV. If the kinetic energy of the electron is E and its de-Broglie wavelength is l, then
A particular hydrogen like atom has its ground state binding "energy 122.4 eV. Its is in ground state. Then :
The electron in a hydrogen atom makes a transition n1 ¾→ n2, where n1 & n2 are the principal quantum numbers of the two states. Assume the Bohr model to be valid. The time period of the electron in the initial state is eight times that in the final state. The possible values of n1 & n2 are :
A beam of ultraviolet light of all wavelengths passes through hydrogen gas at room temperature, in the x-direction. Assume that all photons emitted due to electron transition inside the gas emerge in the y-direction. Let A and B denote the lights emerging from the gas in the x and y directions respectively.
If radiation of allow wavelengths from ultraviolet to infrared is passed through hydrogen agas at room temperature, absorption lines will be observed in the :
In the hydrogen atom, if the reference level of potential energy is assumed to be zero at the ground state level. Choose the incorrect statement.
Statement-1: Figure shows graph of stopping potential and frequency of incident light in photoelectric effect. For values of frequency less than threshold frequecy (v0) stopping potential is negative.
Statement-2 : Lower the value of frequency of incident light (for n > n0) the lower is the maxima of kinetic energy of emitted photoelectrons.
Statement-1: In the process of photo electric emission, all the emitted photoelectrons have same K.E.
Statement-2: According to einstein's photo electric equation KEmax = hn – f.
Statement-1: Work function of aluminum is 4.2 eV. If two photons each of energy 2.5 eV strikes on a piece of aluminum, the photo electric emission does not occur.
Statement-2: In photo electric effect a single photon interacts with a single electron and electron is emitted only if energy of each incident photon is greater then the work function.
Statement-1: An electron and a proton are accelerated through the same potential difference. The deBroglie wavelength associated with the electron is longer.
Statement-2: De-Broglie wavelength associated with a moving particle is l = where, p is the linear momentum and both have same K.E.
Statement-1: Two photons having equal wavelengths have equal linear momenta.
Statement-2: When light shows its photon character, each photon has a linear momentum p = .
A parallel beam of uniform, monochromatic light of wavelength 2640 A has an intensity of 200 W/m2. The number of photons in 1 mm3 of this radiation are ...............
The total energy of the electron in the hydrogen atom in the ground state is -13.6 eV. Which of the following is its kinetic energy in the first excited state?
When a monochromatic point source of light is at a distance of 0.2 m from a photoelectric cell, the cut off voltage and the saturation current are respectively 0.6 volt and 18.0 mA. If the same source is placed 0.6 m away from the photoelectric cell, then find
Q
the stopping potential
When a monochromatic point source of light is at a distance of 0.2 m from a photoelectric cell, the cut off voltage and the saturation current are respectively 0.6 volt and 18.0 mA. If the same source is placed 0.6 m away from the photoelectric cell, then find
Q.
the saturation current
An isolated metal body is illuminated with monochromatic light and is observed to become charged to a steady positive potential 1.0 V with respect to the surrounding. The work function of the metal is 3.0 eV. The frequency of the incident light is ___________________ eV.
663 mW of light from a 540 nm source is incident on the surface of a metal. If only 1 of each 5×109 incident photons in absorbed and causes an electron to be ejected from the surface, the total photocurrent in the circuit is ____________________.
|
9 docs|1272 tests
|