Test: ACT Chemistry Research Summaries Questions - ACT MCQ
10 Questions MCQ Test Science for ACT - Test: ACT Chemistry Research Summaries Questions
Both gases and liquids are considered to be fluids that have individual molecules that move around with kinetic and potential energy. Kinetic energy, defined as the energy related to motion, takes three forms: translational energy that occurs as a molecule moves from position A to position B, rotational energy that occurs as a molecule spins around an imaginary axis at its center of mass, and vibrational energy that occurs as individual atoms in a molecular bond move towards and away from each other. Usually, molecules possess varying combinations of kinetic energy forms. In contrast, potential energy is defined as stored energy that could be released to become kinetic energy. The total energy of a molecule is fixed, meaning that a molecule has some combination of kinetic and potential energies.
Varying amount of kinetic and potential energies define how molecules in a fluid interact with each other. For example, when the kinetic energy of a molecule is high (greater than 1000J), it can no longer interact with neighboring molecules strongly enough to remain a liquid. However, if the potential energies are too high (greater than 1000 J), molecules cannot escape a liquid to become a gas. If the kinetic energy is high and the potential energy is low, molecules tend to become a gas and can be modeled by an equation known as the Ideal Gas Law:
PV=nRT
Where P is the pressure of a gas, V is the volume, n is the number of moles of a gas, R is a constant, and T is temperature in degrees Kelvin.
The Ideal Gas Law perfectly applies to particles with no mass, no intermolecular interactions, and no true volume. However, real molecules do not adhere perfectly to the Ideal Gas Law.
Q. As the potential energy of a molecule decreases, one would expect the kinetic energy to:
Both gases and liquids are considered to be fluids that have individual molecules that move around with kinetic and potential energy. Kinetic energy, defined as the energy related to motion, takes three forms: translational energy that occurs as a molecule moves from position A to position B, rotational energy that occurs as a molecule spins around an imaginary axis at its center of mass, and vibrational energy that occurs as individual atoms in a molecular bond move towards and away from each other. Usually, molecules possess varying combinations of kinetic energy forms. In contrast, potential energy is defined as stored energy that could be released to become kinetic energy. The total energy of a molecule is fixed, meaning that a molecule has some combination of kinetic and potential energies.
Varying amount of kinetic and potential energies define how molecules in a fluid interact with each other. For example, when the kinetic energy of a molecule is high (greater than 1000J), it can no longer interact with neighboring molecules strongly enough to remain a liquid. However, if the potential energies are too high (greater than 1000 J), molecules cannot escape a liquid to become a gas. If the kinetic energy is high and the potential energy is low, molecules tend to become a gas and can be modeled by an equation known as the Ideal Gas Law:
PV=nRT
Where P is the pressure of a gas, V is the volume, n is the number of moles of a gas, R is a constant, and T is temperature in degrees Kelvin.
The Ideal Gas Law perfectly applies to particles with no mass, no intermolecular interactions, and no true volume. However, real molecules do not adhere perfectly to the Ideal Gas Law.
Q. The relationship between total energy, kinetic energy, and potential energy could best be described as:
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Both gases and liquids are considered to be fluids that have individual molecules that move around with kinetic and potential energy. Kinetic energy, defined as the energy related to motion, takes three forms: translational energy that occurs as a molecule moves from position A to position B, rotational energy that occurs as a molecule spins around an imaginary axis at its center of mass, and vibrational energy that occurs as individual atoms in a molecular bond move towards and away from each other. Usually, molecules possess varying combinations of kinetic energy forms. In contrast, potential energy is defined as stored energy that could be released to become kinetic energy. The total energy of a molecule is fixed, meaning that a molecule has some combination of kinetic and potential energies.
Varying amount of kinetic and potential energies define how molecules in a fluid interact with each other. For example, when the kinetic energy of a molecule is high (greater than 1000J), it can no longer interact with neighboring molecules strongly enough to remain a liquid. However, if the potential energies are too high (greater than 1000 J), molecules cannot escape a liquid to become a gas. If the kinetic energy is high and the potential energy is low, molecules tend to become a gas and can be modeled by an equation known as the Ideal Gas Law:
PV=nRT
Where P is the pressure of a gas, V is the volume, n is the number of moles of a gas, R is a constant, and T is temperature in degrees Kelvin.
The Ideal Gas Law perfectly applies to particles with no mass, no intermolecular interactions, and no true volume. However, real molecules do not adhere perfectly to the Ideal Gas Law.
Q. In an oxygen molecule, O2, the two oxygen atoms oscillate about a fixed central point. Which form of kinetic energy is best described here?
Both gases and liquids are considered to be fluids that have individual molecules that move around with kinetic and potential energy. Kinetic energy, defined as the energy related to motion, takes three forms: translational energy that occurs as a molecule moves from position A to position B, rotational energy that occurs as a molecule spins around an imaginary axis at its center of mass, and vibrational energy that occurs as individual atoms in a molecular bond move towards and away from each other. Usually, molecules possess varying combinations of kinetic energy forms. In contrast, potential energy is defined as stored energy that could be released to become kinetic energy. The total energy of a molecule is fixed, meaning that a molecule has some combination of kinetic and potential energies.
Varying amount of kinetic and potential energies define how molecules in a fluid interact with each other. For example, when the kinetic energy of a molecule is high (greater than 1000J), it can no longer interact with neighboring molecules strongly enough to remain a liquid. However, if the potential energies are too high (greater than 1000 J), molecules cannot escape a liquid to become a gas. If the kinetic energy is high and the potential energy is low, molecules tend to become a gas and can be modeled by an equation known as the Ideal Gas Law:
PV=nRT
Where P is the pressure of a gas, V is the volume, n is the number of moles of a gas, R is a constant, and T is temperature in degrees Kelvin.
The Ideal Gas Law perfectly applies to particles with no mass, no intermolecular interactions, and no true volume. However, real molecules do not adhere perfectly to the Ideal Gas Law.
Q. As the kinetic energy of a molecule increases, one would expect the potential energy to:
Both gases and liquids are considered to be fluids that have individual molecules that move around with kinetic and potential energy. Kinetic energy, defined as the energy related to motion, takes three forms: translational energy that occurs as a molecule moves from position A to position B, rotational energy that occurs as a molecule spins around an imaginary axis at its center of mass, and vibrational energy that occurs as individual atoms in a molecular bond move towards and away from each other. Usually, molecules possess varying combinations of kinetic energy forms. In contrast, potential energy is defined as stored energy that could be released to become kinetic energy. The total energy of a molecule is fixed, meaning that a molecule has some combination of kinetic and potential energies.
Varying amount of kinetic and potential energies define how molecules in a fluid interact with each other. For example, when the kinetic energy of a molecule is high (greater than 1000J), it can no longer interact with neighboring molecules strongly enough to remain a liquid. However, if the potential energies are too high (greater than 1000 J), molecules cannot escape a liquid to become a gas. If the kinetic energy is high and the potential energy is low, molecules tend to become a gas and can be modeled by an equation known as the Ideal Gas Law:
PV=nRT
Where P is the pressure of a gas, V is the volume, n is the number of moles of a gas, R is a constant, and T is temperature in degrees Kelvin.
The Ideal Gas Law perfectly applies to particles with no mass, no intermolecular interactions, and no true volume. However, real molecules do not adhere perfectly to the Ideal Gas Law.
Q. An oxygen molecule moving from the left side of a chamber to the right would display what type(s) of kinetic energy?
A student wants to perform an experiment that tests the relationship between the pressure of a gas and the volume it occupies. To perform this experiment, the student places a specific type of gas in a sealed chamber that can change pressure and that can adapt its volume to the gas within it. The chamber also adjusts to the changing pressure such that the temperature (which also has an effect on gas volume) does not change. The following data was obtained:
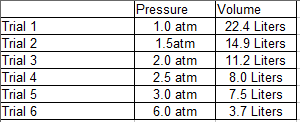
Q. In Trial 4, the chamber used in the experiment malfunctioned and there was a slight decrease in temperature, leading to a faulty reading of volume for Trial 4. Given this information, what can we say about the relationship between the temperature of a gas and its volume?
A student wants to perform an experiment that tests the relationship between the pressure of a gas and the volume it occupies. To perform this experiment, the student places a specific type of gas in a sealed chamber that can change pressure and that can adapt its volume to the gas within it. The chamber also adjusts to the changing pressure such that the temperature (which also has an effect on gas volume) does not change. The following data was obtained:

Q. In a second experiment, the student tries the same experiment described in the pre-question text and uses a different gas for each trial. If the readings for volume yielded the same results, what could be said about the relationship between type of gas and volume?
Two scientists wanted to test the solubility of different substances. Solubility is a measure of how many moles of a given substance (known as the solute) can dissolve in a given volume of another substance (known as the solvent). The solvent can also be thought of as the substance present in greater amount, while the solute can be seen as the substance present in lesser amount. The scientists performed the following experiments to investigate this property.
Experiment 1
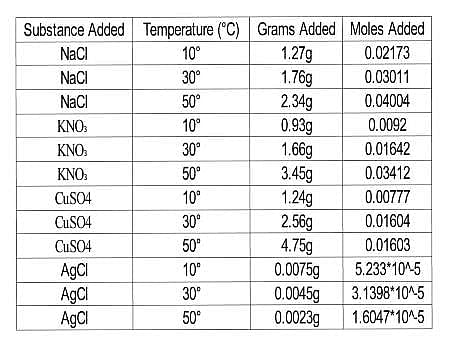
The scientists tested the number of moles of several substances that could be completely dissolved in 50mL of water at various temperatures. They made their solutions by slowly adding amounts of each substance to beakers sitting on a hot plate containing water and a stirring rod until no more of the substance dissolved in the solution. The beakers were weighed before and after the additions and the difference in mass was calculated to be the added mass of the substance. The researchers then calculated the number of moles that dissolved for each trial using the molecular mass and the recorded mass for each trial. Results are recorded in Table 1.
Table 1
Experiment 2
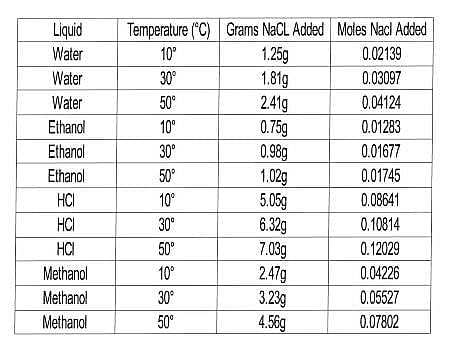
In this experiment, the scientists wanted to test the solubility of NaCl in a variety of liquids at several temperatures. Their procedure was similar to that of Experiment 1, but with a range of liquids and only one solid. The results are compiled in Table 2.
Table 2
Q. Which of the following correctly ranks the solutes from Experiment 1 in decreasing order of solubility in water at 50∘C?
Two scientists wanted to test the solubility of different substances. Solubility is a measure of how many moles of a given substance (known as the solute) can dissolve in a given volume of another substance (known as the solvent). The solvent can also be thought of as the substance present in greater amount, while the solute can be seen as the substance present in lesser amount. The scientists performed the following experiments to investigate this property.
Experiment 1
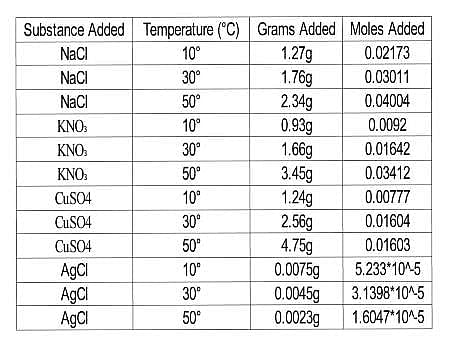
The scientists tested the number of moles of several substances that could be completely dissolved in 50mL of water at various temperatures. They made their solutions by slowly adding amounts of each substance to beakers sitting on a hot plate containing water and a stirring rod until no more of the substance dissolved in the solution. The beakers were weighed before and after the additions and the difference in mass was calculated to be the added mass of the substance. The researchers then calculated the number of moles that dissolved for each trial using the molecular mass and the recorded mass for each trial. Results are recorded in Table 1.
Table 1
Experiment 2
In this experiment, the scientists wanted to test the solubility of NaCl in a variety of liquids at several temperatures. Their procedure was similar to that of Experiment 1, but with a range of liquids and only one solid. The results are compiled in Table 2.
Table 2
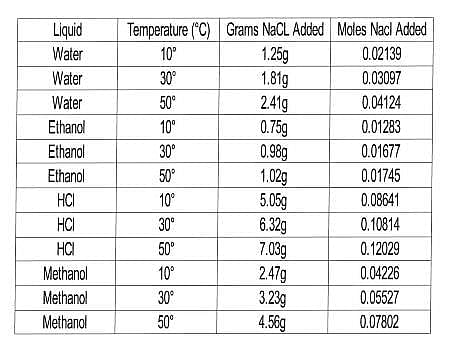
Q. In Experiment 2, which of the following combinations of temperature and solvent dissolved the greatest number of moles of NaCl?
An experiment was carried out measuring the boiling point and freezing point of an unknown organic liquid (Sample X) with varying levels of salt dissolved within it. The experiment was conducted in the following manner:
First, the experimenter measured the boiling point and freezing point of Sample X.
Next, a known quantity of salt was dissolved into the sample and then the boiling point and freezing points were measured again.
The results of the experiment are outlined in the table below:
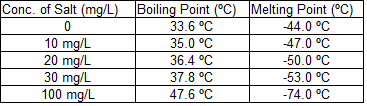
Q. What can we say is the relationship between concentration of salt in Sample X and the boiling point of Sample X?
|
486 videos|517 docs|337 tests
|

















