Test: Gas Laws - ACT MCQ
10 Questions MCQ Test Science for ACT - Test: Gas Laws
At 22 degree Celsius a gas consists of pressure 1.1 bars then what is the temperature when the gas consists a pressure of 2.2 bars?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
There is a balloon filled with a gas at 26-degree centigrade and has a volume of about 2 liters when the balloon is taken to a place which is at 39-degree centigrade, what would be the volume of the gas that is inside the balloon?
There is a ball that will burst if the pressure exceeds 0.12 bars. The pressure of the gas is 1 bar and the volume is 2.5 liters. What can be the maximum volume that the ball can be expanded?
What is the shape of the graph that is drawn between pressure and volume?
When a graph is drawn between the pressure and temperature of the gas it is known as _________
By observing the below-given figure which of the options do you think is the correct one?
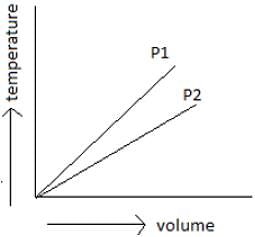
How much does the volume of the gas increase if we increase the temperature by 1 Degree?
What is the name of the graph that is drawn, when the temperature is kept constant?
At a constant temperature, the pressure of a gas is given as one atmospheric pressure and 5 liters. When the atmospheric pressure is increased to 2 atm, then what is the volume of the gas?
|
486 videos|517 docs|337 tests
|

















