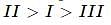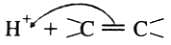Morrison & Boyd Test: Some Basic Principles - NEET MCQ
25 Questions MCQ Test Topic-wise MCQ Tests for NEET - Morrison & Boyd Test: Some Basic Principles
Write the state of hybridisation of carbon in H2C=O.
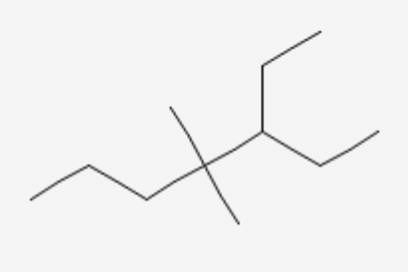
What is the correct order of decreasing stability of the following cations.
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
C4H10O represents methoxypropane (CH3OC3H7) and ethoxyethane (C2H5O C2H5)
Electrophilic addition reactions proceed in two steps. The first step involves the addition of an electrophile. Name the type of intermediate formed in the first step of the following addition reaction.
H3C−−−HC = CH2+ H+→?
During estimation of nitrogen present in an organic compound by Kjeldahl’s method, the ammonia evolved from 0.5 g of the compound, neutralized 10 mL of 1 M H2SO4.Find out the percentage of nitrogen in the compound.
What is the type of hybridisation of each carbon in the compound (CH3)2CO?
The organic compound given below is one of the four options given below. Choose the most appropriate one.
In general, in the molecules containing multiple bonds,_____ provide the most reactive centres. Choose the most appropriate one.
Which of the following is the correct IUPAC name?
In a π (pi) bond formation, is necessary for one of the below criteria. Choose the appropriate one.
Alkyl radical (Methyl, Ethyl, Isopropyl, Tert-butyl free radical) stability in the increasing order is:
The order of decreasing priority for some functional groups in the naming of an organic compound is:
Covalent bond can undergo fission in two different ways. The correct representation involving a heterolytic fission of CH3−−−Br is
When inductive and electromeric effects operate in opposite directions, what happens?
In general, in the molecules containing multiple bonds,_____ provide the most reactive centres. Choose the most appropriate one.
The nitrogen containing organic compound, when heated with copper oxide in an atmosphere of carbon dioxide, yields:
Which of the following statement is not correct for a nucleophile?
The addition of HCl to an alkene proceeds in two steps. The first step is the attack of H+ ion to portion which can be shown as
What does the following diagram showing Lewis structures depict?
Which of the following compounds contain all the carbon atoms in the same hybridisation state?
A known mass of an organic compound is heated with fuming nitric acid in the presence of silver nitrate is:
For testing halogens in an organic compound with AgNO3 solution, sodium extract (Lassaigne’s test) is acidified with dilute HNO3.What will happen if a student acidifies the extract with dilute H2SO4 in place of dilute HNO3?
Nucleophile is a species that should have:
Delocalisation of σ electrons of C -H bond of an alkyl group directly attached to an atom of unsaturated system or to an atom with an unshared p orbital is called :
|
9 docs|1272 tests
|


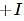 effect The groups showing
effect The groups showing 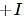 effect are
effect are 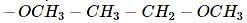 thus the correct stability order of the given carbocations is
thus the correct stability order of the given carbocations is