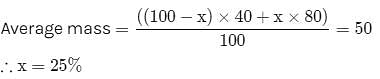RC Mukherjee Test: Mole Concept - NEET MCQ
14 Questions MCQ Test Topic-wise MCQ Tests for NEET - RC Mukherjee Test: Mole Concept
Vapour density of a gas is 22. Its molecular mass will be:
What is the mass of 0.20 mole of C2H5OH (ethanol)?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Consider the reaction between hydrogen and oxygen gases to form water. Which of the following is/are not conserved in the reaction?
2H2(g) + O2(g) → 2H2O(l)
2H2(g) + O2(g) → 2H2O(l)
Mass of sucrose C12H22O11 produced by mixing 84 gm of carbon, 12 gm of hydrogen and 56 lit. O2 at 1 atm & 273 K according to given reaction, is C(s) + H2(g) + O2(g) → C12H22O11 (s)
Suppose the elements X and Y combine to form two compounds XY2 and X3Y2. When 0.1 mole of XY2 weighs 10 g and 0.05 mole of X3Y2 weighs 9 g, the atomic weights of X and Y are
Number of atoms present in 52 g of helium is:
(NA is Avogadro’s constant Gram atomic mass of He is 4 g)
The vapour density of a mixture of gas A (Molecular mass = 40) and gas B (Molecular mass = 80) is 25.Then mole % of gas B in the mixture would be
Atomic mass of Cl is 35.5 g. Calculate the mass of 4.50 moles of chlorine gas, Cl2.
Atomic mass of bromine is 80 g. The mass of four moles of molecular bromine (Br2) is:
Which of the following has maximum number of moles?
Molar mass of F2 is 38 g. How many atoms are present in 0.147 mole of F2?
|
9 docs|1272 tests
|