OneTime: Digital SAT Mock Test - 7 - SAT MCQ
30 Questions MCQ Test Digital SAT Mock Test Series 2024 - OneTime: Digital SAT Mock Test - 7
Question based on the following passage and supplementary material.
This passage is adapted from Kevin Drum, “America's Real Criminal Element: Lead" ©2013 Mother Jones.
Experts often suggest that crime resembles
an epidemic. But what kind? Economics
professor Karl Smith has a good rule of thumb
for categorizing epidemics: If it spreads along
(5) lines of communication, he says, the cause is
information. Think Bieber Fever.* If it travels
along major transportation routes, the cause is
microbial. Think influenza. If it spreads out like
a fan, the cause is an insect. Think malaria. But
(10) if it's everywhere, all at once—as both the rise of
crime in the '60s and '70s and the fall of crime in
the '90s seemed to be—the cause is a molecule.
A molecule? That sounds crazy. What
molecule could be responsible for a steep and
(15) sudden decline in violent crime?
Well, here's one possibility: Pb(CH2CH3)4.
In 1994, Rick Nevin was a consultant
working for the US Department of Housing and
Urban Development on the costs and benefits of
(20) removing lead paint from old houses. A growing
body of research had linked lead exposure in
small children with a whole raft of complications
later in life, including lower IQ, hyperactivity,
behavioral problems, and learning disabilities.
(25) A recent study had also suggested a link
between childhood lead exposure and juvenile
delinquency later on. Maybe reducing lead
exposure had an effect on violent crime too?
That tip took Nevin in a different direction. The
(30) biggest source of lead in the postwar era, it turns
out, wasn't paint, but leaded gasoline. If you chart
the rise and fall of atmospheric lead caused by
the rise and fall of leaded gasoline consumption,
you get an upside-down U. Lead emissions from
(35) tailpipes rose steadily from the early '40s through
the early '70s, nearly quadrupling over that period.
Then, as unleaded gasoline began to replace leaded
gasoline, emissions plummeted.
Intriguingly, violent crime rates followed the
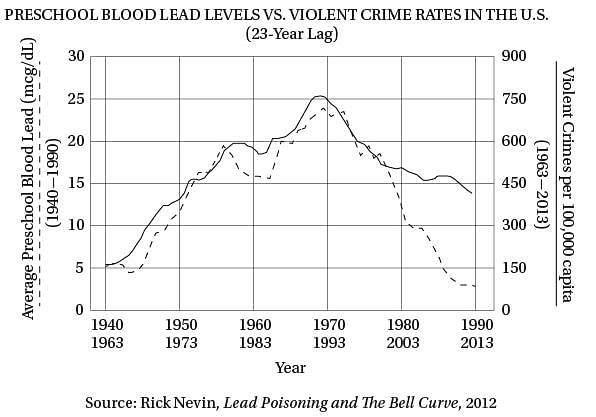
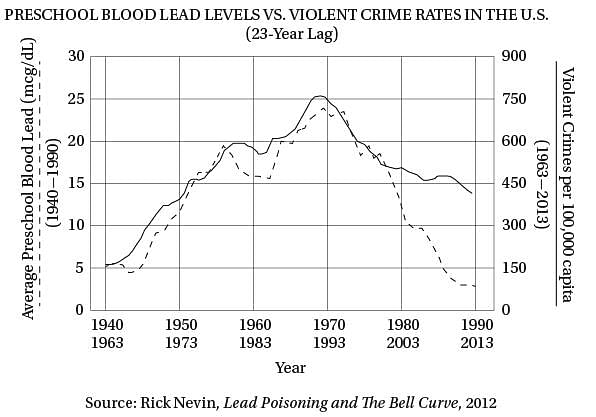
(40) same upside-down U pattern (see the graph). The
only thing different was the time period. Crime
rates rose dramatically in the '60s through the
'80s, and then began dropping steadily starting
in the early '90s. The two curves looked eerily
(45) identical, but were offset by about 20 years.
So Nevin dug up detailed data on lead
emissions and crime rates to see if the similarity
of the curves was as good as it seemed. It turned
out to be even better. In a 2000 paper he concluded
(50) that if you add a lag time of 23 years, lead
emissions from automobiles explain 90 percent of
the variation in violent crime in America. Toddlers
who ingested high levels of lead in the '40s and
'50s really were more likely to become violent
(55) criminals in the '60s, '70s, and '80s.
And with that we have our molecule: tetra-
ethyl lead, the gasoline additive invented by
General Motors in the 1920s to prevent knocking
and pinging in high-performance engines. As
(60) auto sales boomed after World War II, and drivers
in powerful new cars increasingly asked service
station attendants to “fill 'er up with ethyl,” they
were unwittingly creating a crime wave two
decades later.
(65) It was an exciting conjecture, and it
prompted an immediate wave of . . . nothing.
Nevin's paper was almost completely ignored,
and in one sense it's easy to see why—Nevin is
an economist, not a criminologist, and his paper
(70) was published in Environmental Research, not a
journal with a big readership in the criminology
community. What's more, a single correlation
between two curves isn't all that impressive,
econometrically speaking. Sales of vinyl LPs rose
(75) in the postwar period too, and then declined in
the '80s and '90s. No matter how good the fit, if
you only have a single correlation it might just be
a coincidence. You need to do something more to
establish causality.
(80) So in 2007, Nevin collected lead data and
crime data for Australia, Canada, Great Britain,
Finland, France, Italy, New Zealand and West
Germany. Every time, the two curves fit each other
astonishingly well.
(85) The gasoline lead hypothesis helps explain
some things we might not have realized even
needed explaining. For example, murder rates
have always been higher in big cities than in
towns and small cities. Nevin suggests that,
(90) because big cities have lots of cars in a small
area, they also had high densities of atmospheric
lead during the postwar era. But as lead levels
in gasoline decreased, the differences between
big and small cities largely went away. And guess
(95) what? The difference in murder rates went away
too. Today, homicide rates are similar in cities
of all sizes. It may be that violent crime isn't an
inevitable consequence of being a big city after all.
*Enthusiasm for the music and person of Justin Bieber.
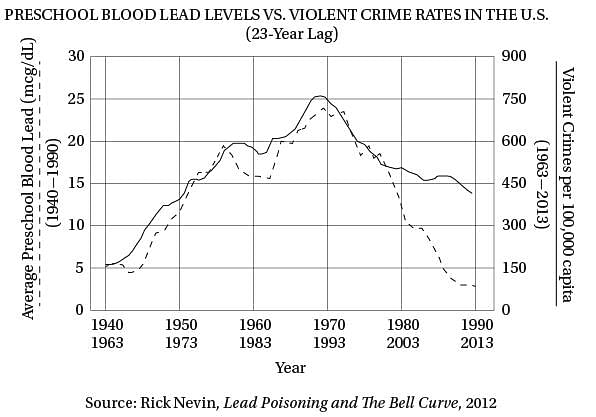
Q. In the first paragraph, Karl Smith’s work is presented primarily as
This passage is adapted from Kevin Drum, “America's Real Criminal Element: Lead" ©2013 Mother Jones.
Experts often suggest that crime resembles
an epidemic. But what kind? Economics
professor Karl Smith has a good rule of thumb
for categorizing epidemics: If it spreads along
(5) lines of communication, he says, the cause is
information. Think Bieber Fever.* If it travels
along major transportation routes, the cause is
microbial. Think influenza. If it spreads out like
a fan, the cause is an insect. Think malaria. But
(10) if it's everywhere, all at once—as both the rise of
crime in the '60s and '70s and the fall of crime in
the '90s seemed to be—the cause is a molecule.
A molecule? That sounds crazy. What
molecule could be responsible for a steep and
(15) sudden decline in violent crime?
Well, here's one possibility: Pb(CH2CH3)4.
In 1994, Rick Nevin was a consultant
working for the US Department of Housing and
Urban Development on the costs and benefits of
(20) removing lead paint from old houses. A growing
body of research had linked lead exposure in
small children with a whole raft of complications
later in life, including lower IQ, hyperactivity,
behavioral problems, and learning disabilities.
(25) A recent study had also suggested a link
between childhood lead exposure and juvenile
delinquency later on. Maybe reducing lead
exposure had an effect on violent crime too?
That tip took Nevin in a different direction. The
(30) biggest source of lead in the postwar era, it turns
out, wasn't paint, but leaded gasoline. If you chart
the rise and fall of atmospheric lead caused by
the rise and fall of leaded gasoline consumption,
you get an upside-down U. Lead emissions from
(35) tailpipes rose steadily from the early '40s through
the early '70s, nearly quadrupling over that period.
Then, as unleaded gasoline began to replace leaded
gasoline, emissions plummeted.
Intriguingly, violent crime rates followed the
(40) same upside-down U pattern (see the graph). The
only thing different was the time period. Crime
rates rose dramatically in the '60s through the
'80s, and then began dropping steadily starting
in the early '90s. The two curves looked eerily
(45) identical, but were offset by about 20 years.
So Nevin dug up detailed data on lead
emissions and crime rates to see if the similarity
of the curves was as good as it seemed. It turned
out to be even better. In a 2000 paper he concluded
(50) that if you add a lag time of 23 years, lead
emissions from automobiles explain 90 percent of
the variation in violent crime in America. Toddlers
who ingested high levels of lead in the '40s and
'50s really were more likely to become violent
(55) criminals in the '60s, '70s, and '80s.
And with that we have our molecule: tetra-
ethyl lead, the gasoline additive invented by
General Motors in the 1920s to prevent knocking
and pinging in high-performance engines. As
(60) auto sales boomed after World War II, and drivers
in powerful new cars increasingly asked service
station attendants to “fill 'er up with ethyl,” they
were unwittingly creating a crime wave two
decades later.
(65) It was an exciting conjecture, and it
prompted an immediate wave of . . . nothing.
Nevin's paper was almost completely ignored,
and in one sense it's easy to see why—Nevin is
an economist, not a criminologist, and his paper
(70) was published in Environmental Research, not a
journal with a big readership in the criminology
community. What's more, a single correlation
between two curves isn't all that impressive,
econometrically speaking. Sales of vinyl LPs rose
(75) in the postwar period too, and then declined in
the '80s and '90s. No matter how good the fit, if
you only have a single correlation it might just be
a coincidence. You need to do something more to
establish causality.
(80) So in 2007, Nevin collected lead data and
crime data for Australia, Canada, Great Britain,
Finland, France, Italy, New Zealand and West
Germany. Every time, the two curves fit each other
astonishingly well.
(85) The gasoline lead hypothesis helps explain
some things we might not have realized even
needed explaining. For example, murder rates
have always been higher in big cities than in
towns and small cities. Nevin suggests that,
(90) because big cities have lots of cars in a small
area, they also had high densities of atmospheric
lead during the postwar era. But as lead levels
in gasoline decreased, the differences between
big and small cities largely went away. And guess
(95) what? The difference in murder rates went away
too. Today, homicide rates are similar in cities
of all sizes. It may be that violent crime isn't an
inevitable consequence of being a big city after all.
*Enthusiasm for the music and person of Justin Bieber.

Question based on the following passage and supplementary material.
This passage is adapted from Kevin Drum, “America's Real Criminal Element: Lead" ©2013 Mother Jones.
Experts often suggest that crime resembles
an epidemic. But what kind? Economics
professor Karl Smith has a good rule of thumb
for categorizing epidemics: If it spreads along
(5) lines of communication, he says, the cause is
information. Think Bieber Fever.* If it travels
along major transportation routes, the cause is
microbial. Think influenza. If it spreads out like
a fan, the cause is an insect. Think malaria. But
(10) if it's everywhere, all at once—as both the rise of
crime in the '60s and '70s and the fall of crime in
the '90s seemed to be—the cause is a molecule.
A molecule? That sounds crazy. What
molecule could be responsible for a steep and
(15) sudden decline in violent crime?
Well, here's one possibility: Pb(CH2CH3)4.
In 1994, Rick Nevin was a consultant
working for the US Department of Housing and
Urban Development on the costs and benefits of
(20) removing lead paint from old houses. A growing
body of research had linked lead exposure in
small children with a whole raft of complications
later in life, including lower IQ, hyperactivity,
behavioral problems, and learning disabilities.
(25) A recent study had also suggested a link
between childhood lead exposure and juvenile
delinquency later on. Maybe reducing lead
exposure had an effect on violent crime too?
That tip took Nevin in a different direction. The
(30) biggest source of lead in the postwar era, it turns
out, wasn't paint, but leaded gasoline. If you chart
the rise and fall of atmospheric lead caused by
the rise and fall of leaded gasoline consumption,
you get an upside-down U. Lead emissions from
(35) tailpipes rose steadily from the early '40s through
the early '70s, nearly quadrupling over that period.
Then, as unleaded gasoline began to replace leaded
gasoline, emissions plummeted.
Intriguingly, violent crime rates followed the
(40) same upside-down U pattern (see the graph). The
only thing different was the time period. Crime
rates rose dramatically in the '60s through the
'80s, and then began dropping steadily starting
in the early '90s. The two curves looked eerily
(45) identical, but were offset by about 20 years.
So Nevin dug up detailed data on lead
emissions and crime rates to see if the similarity
of the curves was as good as it seemed. It turned
out to be even better. In a 2000 paper he concluded
(50) that if you add a lag time of 23 years, lead
emissions from automobiles explain 90 percent of
the variation in violent crime in America. Toddlers
who ingested high levels of lead in the '40s and
'50s really were more likely to become violent
(55) criminals in the '60s, '70s, and '80s.
And with that we have our molecule: tetra-
ethyl lead, the gasoline additive invented by
General Motors in the 1920s to prevent knocking
and pinging in high-performance engines. As
(60) auto sales boomed after World War II, and drivers
in powerful new cars increasingly asked service
station attendants to “fill 'er up with ethyl,” they
were unwittingly creating a crime wave two
decades later.
(65) It was an exciting conjecture, and it
prompted an immediate wave of . . . nothing.
Nevin's paper was almost completely ignored,
and in one sense it's easy to see why—Nevin is
an economist, not a criminologist, and his paper
(70) was published in Environmental Research, not a
journal with a big readership in the criminology
community. What's more, a single correlation
between two curves isn't all that impressive,
econometrically speaking. Sales of vinyl LPs rose
(75) in the postwar period too, and then declined in
the '80s and '90s. No matter how good the fit, if
you only have a single correlation it might just be
a coincidence. You need to do something more to
establish causality.
(80) So in 2007, Nevin collected lead data and
crime data for Australia, Canada, Great Britain,
Finland, France, Italy, New Zealand and West
Germany. Every time, the two curves fit each other
astonishingly well.
(85) The gasoline lead hypothesis helps explain
some things we might not have realized even
needed explaining. For example, murder rates
have always been higher in big cities than in
towns and small cities. Nevin suggests that,
(90) because big cities have lots of cars in a small
area, they also had high densities of atmospheric
lead during the postwar era. But as lead levels
in gasoline decreased, the differences between
big and small cities largely went away. And guess
(95) what? The difference in murder rates went away
too. Today, homicide rates are similar in cities
of all sizes. It may be that violent crime isn't an
inevitable consequence of being a big city after all.
*Enthusiasm for the music and person of Justin Bieber.
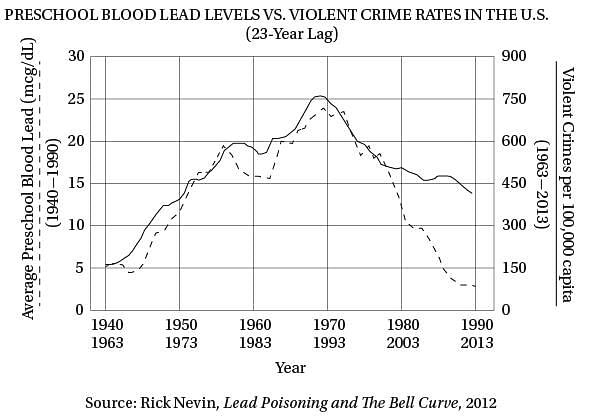
Q. The author suggests that promising research in the social sciences is sometimes ignored because it
This passage is adapted from Kevin Drum, “America's Real Criminal Element: Lead" ©2013 Mother Jones.
Experts often suggest that crime resembles
an epidemic. But what kind? Economics
professor Karl Smith has a good rule of thumb
for categorizing epidemics: If it spreads along
(5) lines of communication, he says, the cause is
information. Think Bieber Fever.* If it travels
along major transportation routes, the cause is
microbial. Think influenza. If it spreads out like
a fan, the cause is an insect. Think malaria. But
(10) if it's everywhere, all at once—as both the rise of
crime in the '60s and '70s and the fall of crime in
the '90s seemed to be—the cause is a molecule.
A molecule? That sounds crazy. What
molecule could be responsible for a steep and
(15) sudden decline in violent crime?
Well, here's one possibility: Pb(CH2CH3)4.
In 1994, Rick Nevin was a consultant
working for the US Department of Housing and
Urban Development on the costs and benefits of
(20) removing lead paint from old houses. A growing
body of research had linked lead exposure in
small children with a whole raft of complications
later in life, including lower IQ, hyperactivity,
behavioral problems, and learning disabilities.
(25) A recent study had also suggested a link
between childhood lead exposure and juvenile
delinquency later on. Maybe reducing lead
exposure had an effect on violent crime too?
That tip took Nevin in a different direction. The
(30) biggest source of lead in the postwar era, it turns
out, wasn't paint, but leaded gasoline. If you chart
the rise and fall of atmospheric lead caused by
the rise and fall of leaded gasoline consumption,
you get an upside-down U. Lead emissions from
(35) tailpipes rose steadily from the early '40s through
the early '70s, nearly quadrupling over that period.
Then, as unleaded gasoline began to replace leaded
gasoline, emissions plummeted.
Intriguingly, violent crime rates followed the
(40) same upside-down U pattern (see the graph). The
only thing different was the time period. Crime
rates rose dramatically in the '60s through the
'80s, and then began dropping steadily starting
in the early '90s. The two curves looked eerily
(45) identical, but were offset by about 20 years.
So Nevin dug up detailed data on lead
emissions and crime rates to see if the similarity
of the curves was as good as it seemed. It turned
out to be even better. In a 2000 paper he concluded
(50) that if you add a lag time of 23 years, lead
emissions from automobiles explain 90 percent of
the variation in violent crime in America. Toddlers
who ingested high levels of lead in the '40s and
'50s really were more likely to become violent
(55) criminals in the '60s, '70s, and '80s.
And with that we have our molecule: tetra-
ethyl lead, the gasoline additive invented by
General Motors in the 1920s to prevent knocking
and pinging in high-performance engines. As
(60) auto sales boomed after World War II, and drivers
in powerful new cars increasingly asked service
station attendants to “fill 'er up with ethyl,” they
were unwittingly creating a crime wave two
decades later.
(65) It was an exciting conjecture, and it
prompted an immediate wave of . . . nothing.
Nevin's paper was almost completely ignored,
and in one sense it's easy to see why—Nevin is
an economist, not a criminologist, and his paper
(70) was published in Environmental Research, not a
journal with a big readership in the criminology
community. What's more, a single correlation
between two curves isn't all that impressive,
econometrically speaking. Sales of vinyl LPs rose
(75) in the postwar period too, and then declined in
the '80s and '90s. No matter how good the fit, if
you only have a single correlation it might just be
a coincidence. You need to do something more to
establish causality.
(80) So in 2007, Nevin collected lead data and
crime data for Australia, Canada, Great Britain,
Finland, France, Italy, New Zealand and West
Germany. Every time, the two curves fit each other
astonishingly well.
(85) The gasoline lead hypothesis helps explain
some things we might not have realized even
needed explaining. For example, murder rates
have always been higher in big cities than in
towns and small cities. Nevin suggests that,
(90) because big cities have lots of cars in a small
area, they also had high densities of atmospheric
lead during the postwar era. But as lead levels
in gasoline decreased, the differences between
big and small cities largely went away. And guess
(95) what? The difference in murder rates went away
too. Today, homicide rates are similar in cities
of all sizes. It may be that violent crime isn't an
inevitable consequence of being a big city after all.
*Enthusiasm for the music and person of Justin Bieber.

| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Question based on the following passage and supplementary material.
This passage is adapted from Kevin Drum, “America's Real Criminal Element: Lead" ©2013 Mother Jones.
Experts often suggest that crime resembles
an epidemic. But what kind? Economics
professor Karl Smith has a good rule of thumb
for categorizing epidemics: If it spreads along
(5) lines of communication, he says, the cause is
information. Think Bieber Fever.* If it travels
along major transportation routes, the cause is
microbial. Think influenza. If it spreads out like
a fan, the cause is an insect. Think malaria. But
(10) if it's everywhere, all at once—as both the rise of
crime in the '60s and '70s and the fall of crime in
the '90s seemed to be—the cause is a molecule.
A molecule? That sounds crazy. What
molecule could be responsible for a steep and
(15) sudden decline in violent crime?
Well, here's one possibility: Pb(CH2CH3)4.
In 1994, Rick Nevin was a consultant
working for the US Department of Housing and
Urban Development on the costs and benefits of
(20) removing lead paint from old houses. A growing
body of research had linked lead exposure in
small children with a whole raft of complications
later in life, including lower IQ, hyperactivity,
behavioral problems, and learning disabilities.
(25) A recent study had also suggested a link
between childhood lead exposure and juvenile
delinquency later on. Maybe reducing lead
exposure had an effect on violent crime too?
That tip took Nevin in a different direction. The
(30) biggest source of lead in the postwar era, it turns
out, wasn't paint, but leaded gasoline. If you chart
the rise and fall of atmospheric lead caused by
the rise and fall of leaded gasoline consumption,
you get an upside-down U. Lead emissions from
(35) tailpipes rose steadily from the early '40s through
the early '70s, nearly quadrupling over that period.
Then, as unleaded gasoline began to replace leaded
gasoline, emissions plummeted.
Intriguingly, violent crime rates followed the
(40) same upside-down U pattern (see the graph). The
only thing different was the time period. Crime
rates rose dramatically in the '60s through the
'80s, and then began dropping steadily starting
in the early '90s. The two curves looked eerily
(45) identical, but were offset by about 20 years.
So Nevin dug up detailed data on lead
emissions and crime rates to see if the similarity
of the curves was as good as it seemed. It turned
out to be even better. In a 2000 paper he concluded
(50) that if you add a lag time of 23 years, lead
emissions from automobiles explain 90 percent of
the variation in violent crime in America. Toddlers
who ingested high levels of lead in the '40s and
'50s really were more likely to become violent
(55) criminals in the '60s, '70s, and '80s.
And with that we have our molecule: tetra-
ethyl lead, the gasoline additive invented by
General Motors in the 1920s to prevent knocking
and pinging in high-performance engines. As
(60) auto sales boomed after World War II, and drivers
in powerful new cars increasingly asked service
station attendants to “fill 'er up with ethyl,” they
were unwittingly creating a crime wave two
decades later.
(65) It was an exciting conjecture, and it
prompted an immediate wave of . . . nothing.
Nevin's paper was almost completely ignored,
and in one sense it's easy to see why—Nevin is
an economist, not a criminologist, and his paper
(70) was published in Environmental Research, not a
journal with a big readership in the criminology
community. What's more, a single correlation
between two curves isn't all that impressive,
econometrically speaking. Sales of vinyl LPs rose
(75) in the postwar period too, and then declined in
the '80s and '90s. No matter how good the fit, if
you only have a single correlation it might just be
a coincidence. You need to do something more to
establish causality.
(80) So in 2007, Nevin collected lead data and
crime data for Australia, Canada, Great Britain,
Finland, France, Italy, New Zealand and West
Germany. Every time, the two curves fit each other
astonishingly well.
(85) The gasoline lead hypothesis helps explain
some things we might not have realized even
needed explaining. For example, murder rates
have always been higher in big cities than in
towns and small cities. Nevin suggests that,
(90) because big cities have lots of cars in a small
area, they also had high densities of atmospheric
lead during the postwar era. But as lead levels
in gasoline decreased, the differences between
big and small cities largely went away. And guess
(95) what? The difference in murder rates went away
too. Today, homicide rates are similar in cities
of all sizes. It may be that violent crime isn't an
inevitable consequence of being a big city after all.
*Enthusiasm for the music and person of Justin Bieber.
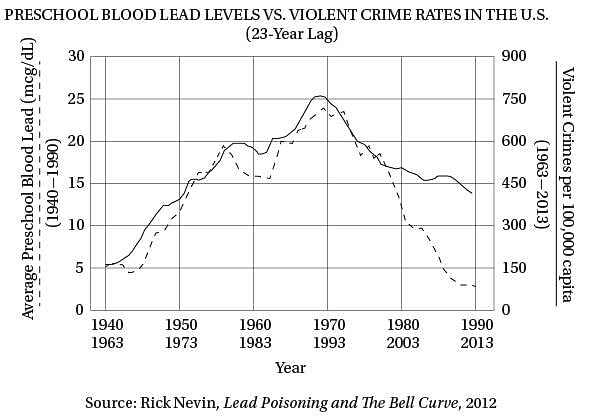
Q. Which of the following provides the strongest evidence for the answer to the previous question?
This passage is adapted from Kevin Drum, “America's Real Criminal Element: Lead" ©2013 Mother Jones.
Experts often suggest that crime resembles
an epidemic. But what kind? Economics
professor Karl Smith has a good rule of thumb
for categorizing epidemics: If it spreads along
(5) lines of communication, he says, the cause is
information. Think Bieber Fever.* If it travels
along major transportation routes, the cause is
microbial. Think influenza. If it spreads out like
a fan, the cause is an insect. Think malaria. But
(10) if it's everywhere, all at once—as both the rise of
crime in the '60s and '70s and the fall of crime in
the '90s seemed to be—the cause is a molecule.
A molecule? That sounds crazy. What
molecule could be responsible for a steep and
(15) sudden decline in violent crime?
Well, here's one possibility: Pb(CH2CH3)4.
In 1994, Rick Nevin was a consultant
working for the US Department of Housing and
Urban Development on the costs and benefits of
(20) removing lead paint from old houses. A growing
body of research had linked lead exposure in
small children with a whole raft of complications
later in life, including lower IQ, hyperactivity,
behavioral problems, and learning disabilities.
(25) A recent study had also suggested a link
between childhood lead exposure and juvenile
delinquency later on. Maybe reducing lead
exposure had an effect on violent crime too?
That tip took Nevin in a different direction. The
(30) biggest source of lead in the postwar era, it turns
out, wasn't paint, but leaded gasoline. If you chart
the rise and fall of atmospheric lead caused by
the rise and fall of leaded gasoline consumption,
you get an upside-down U. Lead emissions from
(35) tailpipes rose steadily from the early '40s through
the early '70s, nearly quadrupling over that period.
Then, as unleaded gasoline began to replace leaded
gasoline, emissions plummeted.
Intriguingly, violent crime rates followed the
(40) same upside-down U pattern (see the graph). The
only thing different was the time period. Crime
rates rose dramatically in the '60s through the
'80s, and then began dropping steadily starting
in the early '90s. The two curves looked eerily
(45) identical, but were offset by about 20 years.
So Nevin dug up detailed data on lead
emissions and crime rates to see if the similarity
of the curves was as good as it seemed. It turned
out to be even better. In a 2000 paper he concluded
(50) that if you add a lag time of 23 years, lead
emissions from automobiles explain 90 percent of
the variation in violent crime in America. Toddlers
who ingested high levels of lead in the '40s and
'50s really were more likely to become violent
(55) criminals in the '60s, '70s, and '80s.
And with that we have our molecule: tetra-
ethyl lead, the gasoline additive invented by
General Motors in the 1920s to prevent knocking
and pinging in high-performance engines. As
(60) auto sales boomed after World War II, and drivers
in powerful new cars increasingly asked service
station attendants to “fill 'er up with ethyl,” they
were unwittingly creating a crime wave two
decades later.
(65) It was an exciting conjecture, and it
prompted an immediate wave of . . . nothing.
Nevin's paper was almost completely ignored,
and in one sense it's easy to see why—Nevin is
an economist, not a criminologist, and his paper
(70) was published in Environmental Research, not a
journal with a big readership in the criminology
community. What's more, a single correlation
between two curves isn't all that impressive,
econometrically speaking. Sales of vinyl LPs rose
(75) in the postwar period too, and then declined in
the '80s and '90s. No matter how good the fit, if
you only have a single correlation it might just be
a coincidence. You need to do something more to
establish causality.
(80) So in 2007, Nevin collected lead data and
crime data for Australia, Canada, Great Britain,
Finland, France, Italy, New Zealand and West
Germany. Every time, the two curves fit each other
astonishingly well.
(85) The gasoline lead hypothesis helps explain
some things we might not have realized even
needed explaining. For example, murder rates
have always been higher in big cities than in
towns and small cities. Nevin suggests that,
(90) because big cities have lots of cars in a small
area, they also had high densities of atmospheric
lead during the postwar era. But as lead levels
in gasoline decreased, the differences between
big and small cities largely went away. And guess
(95) what? The difference in murder rates went away
too. Today, homicide rates are similar in cities
of all sizes. It may be that violent crime isn't an
inevitable consequence of being a big city after all.
*Enthusiasm for the music and person of Justin Bieber.

Question based on the following passage and supplementary material.
This passage is adapted from Kevin Drum, “America's Real Criminal Element: Lead" ©2013 Mother Jones.
Experts often suggest that crime resembles
an epidemic. But what kind? Economics
professor Karl Smith has a good rule of thumb
for categorizing epidemics: If it spreads along
(5) lines of communication, he says, the cause is
information. Think Bieber Fever.* If it travels
along major transportation routes, the cause is
microbial. Think influenza. If it spreads out like
a fan, the cause is an insect. Think malaria. But
(10) if it's everywhere, all at once—as both the rise of
crime in the '60s and '70s and the fall of crime in
the '90s seemed to be—the cause is a molecule.
A molecule? That sounds crazy. What
molecule could be responsible for a steep and
(15) sudden decline in violent crime?
Well, here's one possibility: Pb(CH2CH3)4.
In 1994, Rick Nevin was a consultant
working for the US Department of Housing and
Urban Development on the costs and benefits of
(20) removing lead paint from old houses. A growing
body of research had linked lead exposure in
small children with a whole raft of complications
later in life, including lower IQ, hyperactivity,
behavioral problems, and learning disabilities.
(25) A recent study had also suggested a link
between childhood lead exposure and juvenile
delinquency later on. Maybe reducing lead
exposure had an effect on violent crime too?
That tip took Nevin in a different direction. The
(30) biggest source of lead in the postwar era, it turns
out, wasn't paint, but leaded gasoline. If you chart
the rise and fall of atmospheric lead caused by
the rise and fall of leaded gasoline consumption,
you get an upside-down U. Lead emissions from
(35) tailpipes rose steadily from the early '40s through
the early '70s, nearly quadrupling over that period.
Then, as unleaded gasoline began to replace leaded
gasoline, emissions plummeted.
Intriguingly, violent crime rates followed the
(40) same upside-down U pattern (see the graph). The
only thing different was the time period. Crime
rates rose dramatically in the '60s through the
'80s, and then began dropping steadily starting
in the early '90s. The two curves looked eerily
(45) identical, but were offset by about 20 years.
So Nevin dug up detailed data on lead
emissions and crime rates to see if the similarity
of the curves was as good as it seemed. It turned
out to be even better. In a 2000 paper he concluded
(50) that if you add a lag time of 23 years, lead
emissions from automobiles explain 90 percent of
the variation in violent crime in America. Toddlers
who ingested high levels of lead in the '40s and
'50s really were more likely to become violent
(55) criminals in the '60s, '70s, and '80s.
And with that we have our molecule: tetra-
ethyl lead, the gasoline additive invented by
General Motors in the 1920s to prevent knocking
and pinging in high-performance engines. As
(60) auto sales boomed after World War II, and drivers
in powerful new cars increasingly asked service
station attendants to “fill 'er up with ethyl,” they
were unwittingly creating a crime wave two
decades later.
(65) It was an exciting conjecture, and it
prompted an immediate wave of . . . nothing.
Nevin's paper was almost completely ignored,
and in one sense it's easy to see why—Nevin is
an economist, not a criminologist, and his paper
(70) was published in Environmental Research, not a
journal with a big readership in the criminology
community. What's more, a single correlation
between two curves isn't all that impressive,
econometrically speaking. Sales of vinyl LPs rose
(75) in the postwar period too, and then declined in
the '80s and '90s. No matter how good the fit, if
you only have a single correlation it might just be
a coincidence. You need to do something more to
establish causality.
(80) So in 2007, Nevin collected lead data and
crime data for Australia, Canada, Great Britain,
Finland, France, Italy, New Zealand and West
Germany. Every time, the two curves fit each other
astonishingly well.
(85) The gasoline lead hypothesis helps explain
some things we might not have realized even
needed explaining. For example, murder rates
have always been higher in big cities than in
towns and small cities. Nevin suggests that,
(90) because big cities have lots of cars in a small
area, they also had high densities of atmospheric
lead during the postwar era. But as lead levels
in gasoline decreased, the differences between
big and small cities largely went away. And guess
(95) what? The difference in murder rates went away
too. Today, homicide rates are similar in cities
of all sizes. It may be that violent crime isn't an
inevitable consequence of being a big city after all.
*Enthusiasm for the music and person of Justin Bieber.
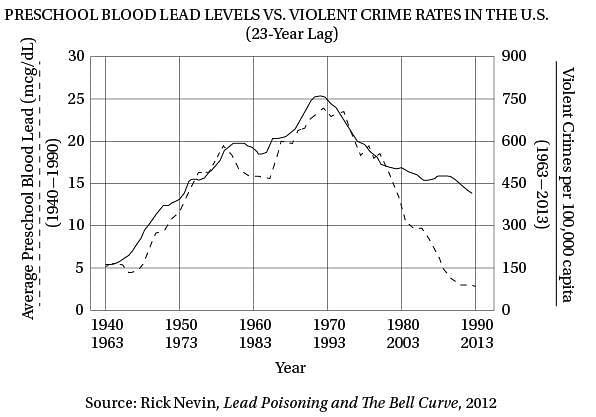
Q. According to the graph for which of the following time periods was the percent increase in per capita violent crime the greatest?
Question based on the following passage and supplementary material.
This passage is adapted from Kevin Drum, “America's Real Criminal Element: Lead" ©2013 Mother Jones.
Experts often suggest that crime resembles
an epidemic. But what kind? Economics
professor Karl Smith has a good rule of thumb
for categorizing epidemics: If it spreads along
(5) lines of communication, he says, the cause is
information. Think Bieber Fever.* If it travels
along major transportation routes, the cause is
microbial. Think influenza. If it spreads out like
a fan, the cause is an insect. Think malaria. But
(10) if it's everywhere, all at once—as both the rise of
crime in the '60s and '70s and the fall of crime in
the '90s seemed to be—the cause is a molecule.
A molecule? That sounds crazy. What
molecule could be responsible for a steep and
(15) sudden decline in violent crime?
Well, here's one possibility: Pb(CH2CH3)4.
In 1994, Rick Nevin was a consultant
working for the US Department of Housing and
Urban Development on the costs and benefits of
(20) removing lead paint from old houses. A growing
body of research had linked lead exposure in
small children with a whole raft of complications
later in life, including lower IQ, hyperactivity,
behavioral problems, and learning disabilities.
(25) A recent study had also suggested a link
between childhood lead exposure and juvenile
delinquency later on. Maybe reducing lead
exposure had an effect on violent crime too?
That tip took Nevin in a different direction. The
(30) biggest source of lead in the postwar era, it turns
out, wasn't paint, but leaded gasoline. If you chart
the rise and fall of atmospheric lead caused by
the rise and fall of leaded gasoline consumption,
you get an upside-down U. Lead emissions from
(35) tailpipes rose steadily from the early '40s through
the early '70s, nearly quadrupling over that period.
Then, as unleaded gasoline began to replace leaded
gasoline, emissions plummeted.
Intriguingly, violent crime rates followed the
(40) same upside-down U pattern (see the graph). The
only thing different was the time period. Crime
rates rose dramatically in the '60s through the
'80s, and then began dropping steadily starting
in the early '90s. The two curves looked eerily
(45) identical, but were offset by about 20 years.
So Nevin dug up detailed data on lead
emissions and crime rates to see if the similarity
of the curves was as good as it seemed. It turned
out to be even better. In a 2000 paper he concluded
(50) that if you add a lag time of 23 years, lead
emissions from automobiles explain 90 percent of
the variation in violent crime in America. Toddlers
who ingested high levels of lead in the '40s and
'50s really were more likely to become violent
(55) criminals in the '60s, '70s, and '80s.
And with that we have our molecule: tetra-
ethyl lead, the gasoline additive invented by
General Motors in the 1920s to prevent knocking
and pinging in high-performance engines. As
(60) auto sales boomed after World War II, and drivers
in powerful new cars increasingly asked service
station attendants to “fill 'er up with ethyl,” they
were unwittingly creating a crime wave two
decades later.
(65) It was an exciting conjecture, and it
prompted an immediate wave of . . . nothing.
Nevin's paper was almost completely ignored,
and in one sense it's easy to see why—Nevin is
an economist, not a criminologist, and his paper
(70) was published in Environmental Research, not a
journal with a big readership in the criminology
community. What's more, a single correlation
between two curves isn't all that impressive,
econometrically speaking. Sales of vinyl LPs rose
(75) in the postwar period too, and then declined in
the '80s and '90s. No matter how good the fit, if
you only have a single correlation it might just be
a coincidence. You need to do something more to
establish causality.
(80) So in 2007, Nevin collected lead data and
crime data for Australia, Canada, Great Britain,
Finland, France, Italy, New Zealand and West
Germany. Every time, the two curves fit each other
astonishingly well.
(85) The gasoline lead hypothesis helps explain
some things we might not have realized even
needed explaining. For example, murder rates
have always been higher in big cities than in
towns and small cities. Nevin suggests that,
(90) because big cities have lots of cars in a small
area, they also had high densities of atmospheric
lead during the postwar era. But as lead levels
in gasoline decreased, the differences between
big and small cities largely went away. And guess
(95) what? The difference in murder rates went away
too. Today, homicide rates are similar in cities
of all sizes. It may be that violent crime isn't an
inevitable consequence of being a big city after all.
*Enthusiasm for the music and person of Justin Bieber.
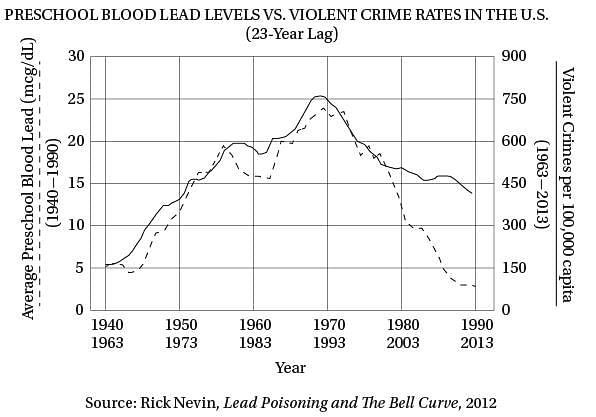
Q. According to the graph, which decade of violent crime statistics provides the LEAST support to Rick Nevin’s hypothesis?
Question based on the following passage and supplementary material.
This passage is adapted from Kevin Drum, “America's Real Criminal Element: Lead" ©2013 Mother Jones.
Experts often suggest that crime resembles
an epidemic. But what kind? Economics
professor Karl Smith has a good rule of thumb
for categorizing epidemics: If it spreads along
(5) lines of communication, he says, the cause is
information. Think Bieber Fever.* If it travels
along major transportation routes, the cause is
microbial. Think influenza. If it spreads out like
a fan, the cause is an insect. Think malaria. But
(10) if it's everywhere, all at once—as both the rise of
crime in the '60s and '70s and the fall of crime in
the '90s seemed to be—the cause is a molecule.
A molecule? That sounds crazy. What
molecule could be responsible for a steep and
(15) sudden decline in violent crime?
Well, here's one possibility: Pb(CH2CH3)4.
In 1994, Rick Nevin was a consultant
working for the US Department of Housing and
Urban Development on the costs and benefits of
(20) removing lead paint from old houses. A growing
body of research had linked lead exposure in
small children with a whole raft of complications
later in life, including lower IQ, hyperactivity,
behavioral problems, and learning disabilities.
(25) A recent study had also suggested a link
between childhood lead exposure and juvenile
delinquency later on. Maybe reducing lead
exposure had an effect on violent crime too?
That tip took Nevin in a different direction. The
(30) biggest source of lead in the postwar era, it turns
out, wasn't paint, but leaded gasoline. If you chart
the rise and fall of atmospheric lead caused by
the rise and fall of leaded gasoline consumption,
you get an upside-down U. Lead emissions from
(35) tailpipes rose steadily from the early '40s through
the early '70s, nearly quadrupling over that period.
Then, as unleaded gasoline began to replace leaded
gasoline, emissions plummeted.
Intriguingly, violent crime rates followed the
(40) same upside-down U pattern (see the graph). The
only thing different was the time period. Crime
rates rose dramatically in the '60s through the
'80s, and then began dropping steadily starting
in the early '90s. The two curves looked eerily
(45) identical, but were offset by about 20 years.
So Nevin dug up detailed data on lead
emissions and crime rates to see if the similarity
of the curves was as good as it seemed. It turned
out to be even better. In a 2000 paper he concluded
(50) that if you add a lag time of 23 years, lead
emissions from automobiles explain 90 percent of
the variation in violent crime in America. Toddlers
who ingested high levels of lead in the '40s and
'50s really were more likely to become violent
(55) criminals in the '60s, '70s, and '80s.
And with that we have our molecule: tetra-
ethyl lead, the gasoline additive invented by
General Motors in the 1920s to prevent knocking
and pinging in high-performance engines. As
(60) auto sales boomed after World War II, and drivers
in powerful new cars increasingly asked service
station attendants to “fill 'er up with ethyl,” they
were unwittingly creating a crime wave two
decades later.
(65) It was an exciting conjecture, and it
prompted an immediate wave of . . . nothing.
Nevin's paper was almost completely ignored,
and in one sense it's easy to see why—Nevin is
an economist, not a criminologist, and his paper
(70) was published in Environmental Research, not a
journal with a big readership in the criminology
community. What's more, a single correlation
between two curves isn't all that impressive,
econometrically speaking. Sales of vinyl LPs rose
(75) in the postwar period too, and then declined in
the '80s and '90s. No matter how good the fit, if
you only have a single correlation it might just be
a coincidence. You need to do something more to
establish causality.
(80) So in 2007, Nevin collected lead data and
crime data for Australia, Canada, Great Britain,
Finland, France, Italy, New Zealand and West
Germany. Every time, the two curves fit each other
astonishingly well.
(85) The gasoline lead hypothesis helps explain
some things we might not have realized even
needed explaining. For example, murder rates
have always been higher in big cities than in
towns and small cities. Nevin suggests that,
(90) because big cities have lots of cars in a small
area, they also had high densities of atmospheric
lead during the postwar era. But as lead levels
in gasoline decreased, the differences between
big and small cities largely went away. And guess
(95) what? The difference in murder rates went away
too. Today, homicide rates are similar in cities
of all sizes. It may be that violent crime isn't an
inevitable consequence of being a big city after all.
*Enthusiasm for the music and person of Justin Bieber.
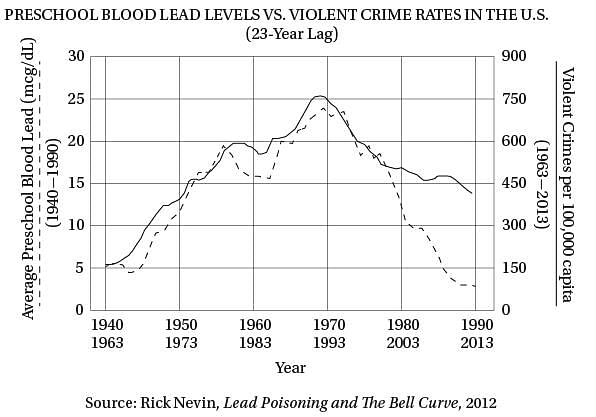
Q. The author mentions “sales of vinyl LPs” (line 74) primarily as an example of
Question based on the following passage and supplementary material.
This passage is adapted from Kevin Drum, “America's Real Criminal Element: Lead" ©2013 Mother Jones.
Experts often suggest that crime resembles
an epidemic. But what kind? Economics
professor Karl Smith has a good rule of thumb
for categorizing epidemics: If it spreads along
(5) lines of communication, he says, the cause is
information. Think Bieber Fever.* If it travels
along major transportation routes, the cause is
microbial. Think influenza. If it spreads out like
a fan, the cause is an insect. Think malaria. But
(10) if it's everywhere, all at once—as both the rise of
crime in the '60s and '70s and the fall of crime in
the '90s seemed to be—the cause is a molecule.
A molecule? That sounds crazy. What
molecule could be responsible for a steep and
(15) sudden decline in violent crime?
Well, here's one possibility: Pb(CH2CH3)4.
In 1994, Rick Nevin was a consultant
working for the US Department of Housing and
Urban Development on the costs and benefits of
(20) removing lead paint from old houses. A growing
body of research had linked lead exposure in
small children with a whole raft of complications
later in life, including lower IQ, hyperactivity,
behavioral problems, and learning disabilities.
(25) A recent study had also suggested a link
between childhood lead exposure and juvenile
delinquency later on. Maybe reducing lead
exposure had an effect on violent crime too?
That tip took Nevin in a different direction. The
(30) biggest source of lead in the postwar era, it turns
out, wasn't paint, but leaded gasoline. If you chart
the rise and fall of atmospheric lead caused by
the rise and fall of leaded gasoline consumption,
you get an upside-down U. Lead emissions from
(35) tailpipes rose steadily from the early '40s through
the early '70s, nearly quadrupling over that period.
Then, as unleaded gasoline began to replace leaded
gasoline, emissions plummeted.
Intriguingly, violent crime rates followed the
(40) same upside-down U pattern (see the graph). The
only thing different was the time period. Crime
rates rose dramatically in the '60s through the
'80s, and then began dropping steadily starting
in the early '90s. The two curves looked eerily
(45) identical, but were offset by about 20 years.
So Nevin dug up detailed data on lead
emissions and crime rates to see if the similarity
of the curves was as good as it seemed. It turned
out to be even better. In a 2000 paper he concluded
(50) that if you add a lag time of 23 years, lead
emissions from automobiles explain 90 percent of
the variation in violent crime in America. Toddlers
who ingested high levels of lead in the '40s and
'50s really were more likely to become violent
(55) criminals in the '60s, '70s, and '80s.
And with that we have our molecule: tetra-
ethyl lead, the gasoline additive invented by
General Motors in the 1920s to prevent knocking
and pinging in high-performance engines. As
(60) auto sales boomed after World War II, and drivers
in powerful new cars increasingly asked service
station attendants to “fill 'er up with ethyl,” they
were unwittingly creating a crime wave two
decades later.
(65) It was an exciting conjecture, and it
prompted an immediate wave of . . . nothing.
Nevin's paper was almost completely ignored,
and in one sense it's easy to see why—Nevin is
an economist, not a criminologist, and his paper
(70) was published in Environmental Research, not a
journal with a big readership in the criminology
community. What's more, a single correlation
between two curves isn't all that impressive,
econometrically speaking. Sales of vinyl LPs rose
(75) in the postwar period too, and then declined in
the '80s and '90s. No matter how good the fit, if
you only have a single correlation it might just be
a coincidence. You need to do something more to
establish causality.
(80) So in 2007, Nevin collected lead data and
crime data for Australia, Canada, Great Britain,
Finland, France, Italy, New Zealand and West
Germany. Every time, the two curves fit each other
astonishingly well.
(85) The gasoline lead hypothesis helps explain
some things we might not have realized even
needed explaining. For example, murder rates
have always been higher in big cities than in
towns and small cities. Nevin suggests that,
(90) because big cities have lots of cars in a small
area, they also had high densities of atmospheric
lead during the postwar era. But as lead levels
in gasoline decreased, the differences between
big and small cities largely went away. And guess
(95) what? The difference in murder rates went away
too. Today, homicide rates are similar in cities
of all sizes. It may be that violent crime isn't an
inevitable consequence of being a big city after all.
*Enthusiasm for the music and person of Justin Bieber.
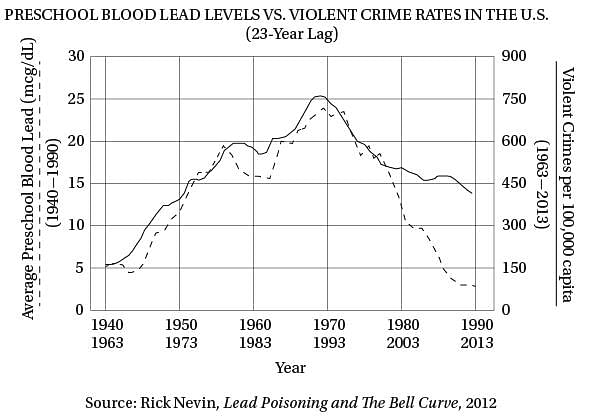
Q. The “complications” in line 22 are
Question based on the following passage and supplementary material.
This passage is adapted from Kevin Drum, “America's Real Criminal Element: Lead" ©2013 Mother Jones.
Experts often suggest that crime resembles
an epidemic. But what kind? Economics
professor Karl Smith has a good rule of thumb
for categorizing epidemics: If it spreads along
(5) lines of communication, he says, the cause is
information. Think Bieber Fever.* If it travels
along major transportation routes, the cause is
microbial. Think influenza. If it spreads out like
a fan, the cause is an insect. Think malaria. But
(10) if it's everywhere, all at once—as both the rise of
crime in the '60s and '70s and the fall of crime in
the '90s seemed to be—the cause is a molecule.
A molecule? That sounds crazy. What
molecule could be responsible for a steep and
(15) sudden decline in violent crime?
Well, here's one possibility: Pb(CH2CH3)4.
In 1994, Rick Nevin was a consultant
working for the US Department of Housing and
Urban Development on the costs and benefits of
(20) removing lead paint from old houses. A growing
body of research had linked lead exposure in
small children with a whole raft of complications
later in life, including lower IQ, hyperactivity,
behavioral problems, and learning disabilities.
(25) A recent study had also suggested a link
between childhood lead exposure and juvenile
delinquency later on. Maybe reducing lead
exposure had an effect on violent crime too?
That tip took Nevin in a different direction. The
(30) biggest source of lead in the postwar era, it turns
out, wasn't paint, but leaded gasoline. If you chart
the rise and fall of atmospheric lead caused by
the rise and fall of leaded gasoline consumption,
you get an upside-down U. Lead emissions from
(35) tailpipes rose steadily from the early '40s through
the early '70s, nearly quadrupling over that period.
Then, as unleaded gasoline began to replace leaded
gasoline, emissions plummeted.
Intriguingly, violent crime rates followed the
(40) same upside-down U pattern (see the graph). The
only thing different was the time period. Crime
rates rose dramatically in the '60s through the
'80s, and then began dropping steadily starting
in the early '90s. The two curves looked eerily
(45) identical, but were offset by about 20 years.
So Nevin dug up detailed data on lead
emissions and crime rates to see if the similarity
of the curves was as good as it seemed. It turned
out to be even better. In a 2000 paper he concluded
(50) that if you add a lag time of 23 years, lead
emissions from automobiles explain 90 percent of
the variation in violent crime in America. Toddlers
who ingested high levels of lead in the '40s and
'50s really were more likely to become violent
(55) criminals in the '60s, '70s, and '80s.
And with that we have our molecule: tetra-
ethyl lead, the gasoline additive invented by
General Motors in the 1920s to prevent knocking
and pinging in high-performance engines. As
(60) auto sales boomed after World War II, and drivers
in powerful new cars increasingly asked service
station attendants to “fill 'er up with ethyl,” they
were unwittingly creating a crime wave two
decades later.
(65) It was an exciting conjecture, and it
prompted an immediate wave of . . . nothing.
Nevin's paper was almost completely ignored,
and in one sense it's easy to see why—Nevin is
an economist, not a criminologist, and his paper
(70) was published in Environmental Research, not a
journal with a big readership in the criminology
community. What's more, a single correlation
between two curves isn't all that impressive,
econometrically speaking. Sales of vinyl LPs rose
(75) in the postwar period too, and then declined in
the '80s and '90s. No matter how good the fit, if
you only have a single correlation it might just be
a coincidence. You need to do something more to
establish causality.
(80) So in 2007, Nevin collected lead data and
crime data for Australia, Canada, Great Britain,
Finland, France, Italy, New Zealand and West
Germany. Every time, the two curves fit each other
astonishingly well.
(85) The gasoline lead hypothesis helps explain
some things we might not have realized even
needed explaining. For example, murder rates
have always been higher in big cities than in
towns and small cities. Nevin suggests that,
(90) because big cities have lots of cars in a small
area, they also had high densities of atmospheric
lead during the postwar era. But as lead levels
in gasoline decreased, the differences between
big and small cities largely went away. And guess
(95) what? The difference in murder rates went away
too. Today, homicide rates are similar in cities
of all sizes. It may be that violent crime isn't an
inevitable consequence of being a big city after all.
*Enthusiasm for the music and person of Justin Bieber.
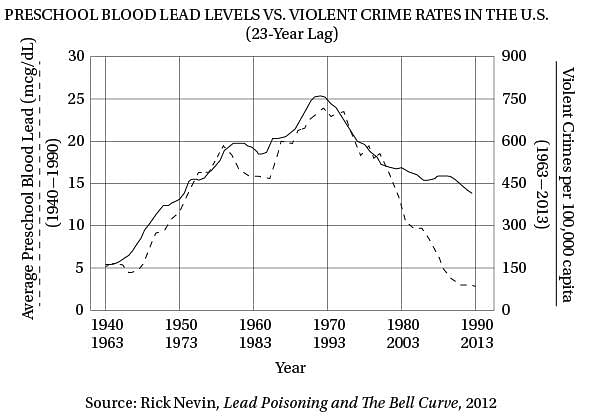
Q. The author regards the “drivers” in line 60 as
Question based on the following passage and supplementary material.
This passage is adapted from Kevin Drum, “America's Real Criminal Element: Lead" ©2013 Mother Jones.
Experts often suggest that crime resembles
an epidemic. But what kind? Economics
professor Karl Smith has a good rule of thumb
for categorizing epidemics: If it spreads along
(5) lines of communication, he says, the cause is
information. Think Bieber Fever.* If it travels
along major transportation routes, the cause is
microbial. Think influenza. If it spreads out like
a fan, the cause is an insect. Think malaria. But
(10) if it's everywhere, all at once—as both the rise of
crime in the '60s and '70s and the fall of crime in
the '90s seemed to be—the cause is a molecule.
A molecule? That sounds crazy. What
molecule could be responsible for a steep and
(15) sudden decline in violent crime?
Well, here's one possibility: Pb(CH2CH3)4.
In 1994, Rick Nevin was a consultant
working for the US Department of Housing and
Urban Development on the costs and benefits of
(20) removing lead paint from old houses. A growing
body of research had linked lead exposure in
small children with a whole raft of complications
later in life, including lower IQ, hyperactivity,
behavioral problems, and learning disabilities.
(25) A recent study had also suggested a link
between childhood lead exposure and juvenile
delinquency later on. Maybe reducing lead
exposure had an effect on violent crime too?
That tip took Nevin in a different direction. The
(30) biggest source of lead in the postwar era, it turns
out, wasn't paint, but leaded gasoline. If you chart
the rise and fall of atmospheric lead caused by
the rise and fall of leaded gasoline consumption,
you get an upside-down U. Lead emissions from
(35) tailpipes rose steadily from the early '40s through
the early '70s, nearly quadrupling over that period.
Then, as unleaded gasoline began to replace leaded
gasoline, emissions plummeted.
Intriguingly, violent crime rates followed the
(40) same upside-down U pattern (see the graph). The
only thing different was the time period. Crime
rates rose dramatically in the '60s through the
'80s, and then began dropping steadily starting
in the early '90s. The two curves looked eerily
(45) identical, but were offset by about 20 years.
So Nevin dug up detailed data on lead
emissions and crime rates to see if the similarity
of the curves was as good as it seemed. It turned
out to be even better. In a 2000 paper he concluded
(50) that if you add a lag time of 23 years, lead
emissions from automobiles explain 90 percent of
the variation in violent crime in America. Toddlers
who ingested high levels of lead in the '40s and
'50s really were more likely to become violent
(55) criminals in the '60s, '70s, and '80s.
And with that we have our molecule: tetra-
ethyl lead, the gasoline additive invented by
General Motors in the 1920s to prevent knocking
and pinging in high-performance engines. As
(60) auto sales boomed after World War II, and drivers
in powerful new cars increasingly asked service
station attendants to “fill 'er up with ethyl,” they
were unwittingly creating a crime wave two
decades later.
(65) It was an exciting conjecture, and it
prompted an immediate wave of . . . nothing.
Nevin's paper was almost completely ignored,
and in one sense it's easy to see why—Nevin is
an economist, not a criminologist, and his paper
(70) was published in Environmental Research, not a
journal with a big readership in the criminology
community. What's more, a single correlation
between two curves isn't all that impressive,
econometrically speaking. Sales of vinyl LPs rose
(75) in the postwar period too, and then declined in
the '80s and '90s. No matter how good the fit, if
you only have a single correlation it might just be
a coincidence. You need to do something more to
establish causality.
(80) So in 2007, Nevin collected lead data and
crime data for Australia, Canada, Great Britain,
Finland, France, Italy, New Zealand and West
Germany. Every time, the two curves fit each other
astonishingly well.
(85) The gasoline lead hypothesis helps explain
some things we might not have realized even
needed explaining. For example, murder rates
have always been higher in big cities than in
towns and small cities. Nevin suggests that,
(90) because big cities have lots of cars in a small
area, they also had high densities of atmospheric
lead during the postwar era. But as lead levels
in gasoline decreased, the differences between
big and small cities largely went away. And guess
(95) what? The difference in murder rates went away
too. Today, homicide rates are similar in cities
of all sizes. It may be that violent crime isn't an
inevitable consequence of being a big city after all.
*Enthusiasm for the music and person of Justin Bieber.
Q. In line 49, “even better” most nearly means
Question based on the following passage and supplementary material.
This passage is adapted from Kevin Drum, “America's Real Criminal Element: Lead" ©2013 Mother Jones.
Experts often suggest that crime resembles
an epidemic. But what kind? Economics
professor Karl Smith has a good rule of thumb
for categorizing epidemics: If it spreads along
(5) lines of communication, he says, the cause is
information. Think Bieber Fever.* If it travels
along major transportation routes, the cause is
microbial. Think influenza. If it spreads out like
a fan, the cause is an insect. Think malaria. But
(10) if it's everywhere, all at once—as both the rise of
crime in the '60s and '70s and the fall of crime in
the '90s seemed to be—the cause is a molecule.
A molecule? That sounds crazy. What
molecule could be responsible for a steep and
(15) sudden decline in violent crime?
Well, here's one possibility: Pb(CH2CH3)4.
In 1994, Rick Nevin was a consultant
working for the US Department of Housing and
Urban Development on the costs and benefits of
(20) removing lead paint from old houses. A growing
body of research had linked lead exposure in
small children with a whole raft of complications
later in life, including lower IQ, hyperactivity,
behavioral problems, and learning disabilities.
(25) A recent study had also suggested a link
between childhood lead exposure and juvenile
delinquency later on. Maybe reducing lead
exposure had an effect on violent crime too?
That tip took Nevin in a different direction. The
(30) biggest source of lead in the postwar era, it turns
out, wasn't paint, but leaded gasoline. If you chart
the rise and fall of atmospheric lead caused by
the rise and fall of leaded gasoline consumption,
you get an upside-down U. Lead emissions from
(35) tailpipes rose steadily from the early '40s through
the early '70s, nearly quadrupling over that period.
Then, as unleaded gasoline began to replace leaded
gasoline, emissions plummeted.
Intriguingly, violent crime rates followed the
(40) same upside-down U pattern (see the graph). The
only thing different was the time period. Crime
rates rose dramatically in the '60s through the
'80s, and then began dropping steadily starting
in the early '90s. The two curves looked eerily
(45) identical, but were offset by about 20 years.
So Nevin dug up detailed data on lead
emissions and crime rates to see if the similarity
of the curves was as good as it seemed. It turned
out to be even better. In a 2000 paper he concluded
(50) that if you add a lag time of 23 years, lead
emissions from automobiles explain 90 percent of
the variation in violent crime in America. Toddlers
who ingested high levels of lead in the '40s and
'50s really were more likely to become violent
(55) criminals in the '60s, '70s, and '80s.
And with that we have our molecule: tetra-
ethyl lead, the gasoline additive invented by
General Motors in the 1920s to prevent knocking
and pinging in high-performance engines. As
(60) auto sales boomed after World War II, and drivers
in powerful new cars increasingly asked service
station attendants to “fill 'er up with ethyl,” they
were unwittingly creating a crime wave two
decades later.
(65) It was an exciting conjecture, and it
prompted an immediate wave of . . . nothing.
Nevin's paper was almost completely ignored,
and in one sense it's easy to see why—Nevin is
an economist, not a criminologist, and his paper
(70) was published in Environmental Research, not a
journal with a big readership in the criminology
community. What's more, a single correlation
between two curves isn't all that impressive,
econometrically speaking. Sales of vinyl LPs rose
(75) in the postwar period too, and then declined in
the '80s and '90s. No matter how good the fit, if
you only have a single correlation it might just be
a coincidence. You need to do something more to
establish causality.
(80) So in 2007, Nevin collected lead data and
crime data for Australia, Canada, Great Britain,
Finland, France, Italy, New Zealand and West
Germany. Every time, the two curves fit each other
astonishingly well.
(85) The gasoline lead hypothesis helps explain
some things we might not have realized even
needed explaining. For example, murder rates
have always been higher in big cities than in
towns and small cities. Nevin suggests that,
(90) because big cities have lots of cars in a small
area, they also had high densities of atmospheric
lead during the postwar era. But as lead levels
in gasoline decreased, the differences between
big and small cities largely went away. And guess
(95) what? The difference in murder rates went away
too. Today, homicide rates are similar in cities
of all sizes. It may be that violent crime isn't an
inevitable consequence of being a big city after all.
*Enthusiasm for the music and person of Justin Bieber.
Q. The final paragraph (lines 85–98) serves primarily to
Question based on the following passages.
Passage 1 is adapted from an essay written by John Aldridge in 1951. ©1951 by John Aldridge. Passage 2 is adapted from Brom Weber, "Ernest Hemingway's Genteel Bullfight," published in The American Novel and the Nineteen Twenties. ©1971 by Hodder Education.
Passage 1
By the time we were old enough to read
Hemingway, he had become legendary. Like
Lord Byron a century earlier, he had learned
to play himself, his own best hero, with superb
(5) conviction. He was Hemingway of the rugged
outdoor grin and the hairy chest posing beside a
lion he had just shot. He was Tarzan Hemingway,
crouching in the African bush with elephant gun
at ready. He was War Correspondent Hemingway
(10) writing a play in the Hotel Florida in Madrid
while thirty fascist shells crashed through
the roof. Later, he was Task Force Hemingway
swathed in ammunition belts and defending
his post singlehandedly against fierce German
(15) attacks.
But even without the legend, the chest
beating, wisecracking pose that was later to
seem so incredibly absurd, his impact upon us
was tremendous. The feeling he gave us was one
(20) of immense expansiveness, freedom and, at the
same time, absolute stability and control. We
could follow him, imitate his cold detachment,
through all the doubts and fears of adolescence
and come out pure and untouched. The words
(25) he put down seemed to us to have been carved
from the living stone of life. They conveyed
exactly the taste, smell and feel of experience as
it was, as it might possibly be. And so we began
unconsciously to translate our own sensations
(30) into their terms and to impose on everything
we did and felt the particular emotions they
aroused in us.
The Hemingway time was a good time to
be young. We had much then that the war later
(35) forced out of us, something far greater than
Hemingway's strong formative influence.
Later writers who lost or got rid of Hemingway
have been able to find nothing to put in his
place. They have rejected his time as untrue
(40) for them only to fail at finding themselves in their
own time. Others, in their embarrassment at the
hold he once had over them, have not profited
by the lessons he had to teach, and still others
were never touched by him at all. These last are
(45) perhaps the real unfortunates, for they have been
denied access to a powerful tradition.
Passage 2
One wonders why Hemingway's greatest
works now seem unable to evoke the same sense
of a tottering world that in the 1920s established
(50) Ernest Hemingway's reputation. These novels
should be speaking to us. Our social structure
is as shaken, our philosophical despair as great,
our everyday experience as unsatisfying. We have
had more war than Hemingway ever dreamed
(55) of. Our violence—physical, emotional, and
intellectual—is not inferior to that of the 1920s.
Yet Hemingway's great novels no longer seem to
penetrate deeply the surface of existence.
One begins to doubt that they ever did so significantly
(60) in the 1920s.
Hemingway's novels indulged the dominant
genteel tradition in American culture while
seeming to repudiate it. They yielded to the
functionalist, technological aesthetic of the
(65) culture instead of resisting in the manner of
Frank Lloyd Wright. Hemingway, in effect, became a
dupe of his culture rather than its moral-aesthetic
conscience. As a consequence, the import of his
work has diminished. There is some evidence
(70) from his stylistic evolution that Hemingway
himself must have felt as much, for Hemingway's
famous stylistic economy frequently seems to
conceal another kind of writer, with much richer
rhetorical resources to hand. So, Death in the
(75) Afternoon (1932), Hemingway's bullfighting
opus and his first book after A Farewell to Arms
(1929), reveals great uneasiness over his earlier
accomplishment. In it, he defends his literary
method with a doctrine of ambiguity: “If a writer
(80) of prose knows enough about what he is writing
about he may omit things that he knows and
the reader, if the writer is writing truly enough,
will have a feeling of those things as strongly as
though the writer had stated them.”
(85) Hemingway made much the same theoretical
point in another way in Death in the Afternoon
apparently believing that a formal reduction of
aesthetic complexity was the only kind of design
that had value.
(90) Perhaps the greatest irony of Death in the
Afternoon is its unmistakably baroque prose,
which Hemingway himself embarrassedly
admitted was “flowery.” Reviewers, unable to
challenge Hemingway's expertise in the art of
(95) bullfighting, noted that its style was “awkward,
tortuous, [and] belligerently clumsy.”
Death in the Afternoon is an extraordinarily
self-indulgent, unruly, clownish, garrulous,
and satiric book, with scrambled chronologies,
(100) willful digressions, mock-scholarly apparatuses,
fictional interludes, and scathing allusions. Its
inflated style can hardly penetrate the fagade, let
alone deflate humanity.
Q. On which topic do the authors of the two passages most strongly disagree?
Question based on the following passages.
Passage 1 is adapted from an essay written by John Aldridge in 1951. ©1951 by John Aldridge. Passage 2 is adapted from Brom Weber, "Ernest Hemingway's Genteel Bullfight," published in The American Novel and the Nineteen Twenties. ©1971 by Hodder Education.
Passage 1
By the time we were old enough to read
Hemingway, he had become legendary. Like
Lord Byron a century earlier, he had learned
to play himself, his own best hero, with superb
(5) conviction. He was Hemingway of the rugged
outdoor grin and the hairy chest posing beside a
lion he had just shot. He was Tarzan Hemingway,
crouching in the African bush with elephant gun
at ready. He was War Correspondent Hemingway
(10) writing a play in the Hotel Florida in Madrid
while thirty fascist shells crashed through
the roof. Later, he was Task Force Hemingway
swathed in ammunition belts and defending
his post singlehandedly against fierce German
(15) attacks.
But even without the legend, the chest
beating, wisecracking pose that was later to
seem so incredibly absurd, his impact upon us
was tremendous. The feeling he gave us was one
(20) of immense expansiveness, freedom and, at the
same time, absolute stability and control. We
could follow him, imitate his cold detachment,
through all the doubts and fears of adolescence
and come out pure and untouched. The words
(25) he put down seemed to us to have been carved
from the living stone of life. They conveyed
exactly the taste, smell and feel of experience as
it was, as it might possibly be. And so we began
unconsciously to translate our own sensations
(30) into their terms and to impose on everything
we did and felt the particular emotions they
aroused in us.
The Hemingway time was a good time to
be young. We had much then that the war later
(35) forced out of us, something far greater than
Hemingway's strong formative influence.
Later writers who lost or got rid of Hemingway
have been able to find nothing to put in his
place. They have rejected his time as untrue
(40) for them only to fail at finding themselves in their
own time. Others, in their embarrassment at the
hold he once had over them, have not profited
by the lessons he had to teach, and still others
were never touched by him at all. These last are
(45) perhaps the real unfortunates, for they have been
denied access to a powerful tradition.
Passage 2
One wonders why Hemingway's greatest
works now seem unable to evoke the same sense
of a tottering world that in the 1920s established
(50) Ernest Hemingway's reputation. These novels
should be speaking to us. Our social structure
is as shaken, our philosophical despair as great,
our everyday experience as unsatisfying. We have
had more war than Hemingway ever dreamed
(55) of. Our violence—physical, emotional, and
intellectual—is not inferior to that of the 1920s.
Yet Hemingway's great novels no longer seem to
penetrate deeply the surface of existence.
One begins to doubt that they ever did so significantly
(60) in the 1920s.
Hemingway's novels indulged the dominant
genteel tradition in American culture while
seeming to repudiate it. They yielded to the
functionalist, technological aesthetic of the
(65) culture instead of resisting in the manner of
Frank Lloyd Wright. Hemingway, in effect, became a
dupe of his culture rather than its moral-aesthetic
conscience. As a consequence, the import of his
work has diminished. There is some evidence
(70) from his stylistic evolution that Hemingway
himself must have felt as much, for Hemingway's
famous stylistic economy frequently seems to
conceal another kind of writer, with much richer
rhetorical resources to hand. So, Death in the
(75) Afternoon (1932), Hemingway's bullfighting
opus and his first book after A Farewell to Arms
(1929), reveals great uneasiness over his earlier
accomplishment. In it, he defends his literary
method with a doctrine of ambiguity: “If a writer
(80) of prose knows enough about what he is writing
about he may omit things that he knows and
the reader, if the writer is writing truly enough,
will have a feeling of those things as strongly as
though the writer had stated them.”
(85) Hemingway made much the same theoretical
point in another way in Death in the Afternoon
apparently believing that a formal reduction of
aesthetic complexity was the only kind of design
that had value.
(90) Perhaps the greatest irony of Death in the
Afternoon is its unmistakably baroque prose,
which Hemingway himself embarrassedly
admitted was “flowery.” Reviewers, unable to
challenge Hemingway's expertise in the art of
(95) bullfighting, noted that its style was “awkward,
tortuous, [and] belligerently clumsy.”
Death in the Afternoon is an extraordinarily
self-indulgent, unruly, clownish, garrulous,
and satiric book, with scrambled chronologies,
(100) willful digressions, mock-scholarly apparatuses,
fictional interludes, and scathing allusions. Its
inflated style can hardly penetrate the fagade, let
alone deflate humanity.
Q. Which pair of sentences provides the strongest evidence for the answer to the previous question?
Question based on the following passages.
Passage 1 is adapted from an essay written by John Aldridge in 1951. ©1951 by John Aldridge. Passage 2 is adapted from Brom Weber, "Ernest Hemingway's Genteel Bullfight," published in The American Novel and the Nineteen Twenties. ©1971 by Hodder Education.
Passage 1
By the time we were old enough to read
Hemingway, he had become legendary. Like
Lord Byron a century earlier, he had learned
to play himself, his own best hero, with superb
(5) conviction. He was Hemingway of the rugged
outdoor grin and the hairy chest posing beside a
lion he had just shot. He was Tarzan Hemingway,
crouching in the African bush with elephant gun
at ready. He was War Correspondent Hemingway
(10) writing a play in the Hotel Florida in Madrid
while thirty fascist shells crashed through
the roof. Later, he was Task Force Hemingway
swathed in ammunition belts and defending
his post singlehandedly against fierce German
(15) attacks.
But even without the legend, the chest
beating, wisecracking pose that was later to
seem so incredibly absurd, his impact upon us
was tremendous. The feeling he gave us was one
(20) of immense expansiveness, freedom and, at the
same time, absolute stability and control. We
could follow him, imitate his cold detachment,
through all the doubts and fears of adolescence
and come out pure and untouched. The words
(25) he put down seemed to us to have been carved
from the living stone of life. They conveyed
exactly the taste, smell and feel of experience as
it was, as it might possibly be. And so we began
unconsciously to translate our own sensations
(30) into their terms and to impose on everything
we did and felt the particular emotions they
aroused in us.
The Hemingway time was a good time to
be young. We had much then that the war later
(35) forced out of us, something far greater than
Hemingway's strong formative influence.
Later writers who lost or got rid of Hemingway
have been able to find nothing to put in his
place. They have rejected his time as untrue
(40) for them only to fail at finding themselves in their
own time. Others, in their embarrassment at the
hold he once had over them, have not profited
by the lessons he had to teach, and still others
were never touched by him at all. These last are
(45) perhaps the real unfortunates, for they have been
denied access to a powerful tradition.
Passage 2
One wonders why Hemingway's greatest
works now seem unable to evoke the same sense
of a tottering world that in the 1920s established
(50) Ernest Hemingway's reputation. These novels
should be speaking to us. Our social structure
is as shaken, our philosophical despair as great,
our everyday experience as unsatisfying. We have
had more war than Hemingway ever dreamed
(55) of. Our violence—physical, emotional, and
intellectual—is not inferior to that of the 1920s.
Yet Hemingway's great novels no longer seem to
penetrate deeply the surface of existence.
One begins to doubt that they ever did so significantly
(60) in the 1920s.
Hemingway's novels indulged the dominant
genteel tradition in American culture while
seeming to repudiate it. They yielded to the
functionalist, technological aesthetic of the
(65) culture instead of resisting in the manner of
Frank Lloyd Wright. Hemingway, in effect, became a
dupe of his culture rather than its moral-aesthetic
conscience. As a consequence, the import of his
work has diminished. There is some evidence
(70) from his stylistic evolution that Hemingway
himself must have felt as much, for Hemingway's
famous stylistic economy frequently seems to
conceal another kind of writer, with much richer
rhetorical resources to hand. So, Death in the
(75) Afternoon (1932), Hemingway's bullfighting
opus and his first book after A Farewell to Arms
(1929), reveals great uneasiness over his earlier
accomplishment. In it, he defends his literary
method with a doctrine of ambiguity: “If a writer
(80) of prose knows enough about what he is writing
about he may omit things that he knows and
the reader, if the writer is writing truly enough,
will have a feeling of those things as strongly as
though the writer had stated them.”
(85) Hemingway made much the same theoretical
point in another way in Death in the Afternoon
apparently believing that a formal reduction of
aesthetic complexity was the only kind of design
that had value.
(90) Perhaps the greatest irony of Death in the
Afternoon is its unmistakably baroque prose,
which Hemingway himself embarrassedly
admitted was “flowery.” Reviewers, unable to
challenge Hemingway's expertise in the art of
(95) bullfighting, noted that its style was “awkward,
tortuous, [and] belligerently clumsy.”
Death in the Afternoon is an extraordinarily
self-indulgent, unruly, clownish, garrulous,
and satiric book, with scrambled chronologies,
(100) willful digressions, mock-scholarly apparatuses,
fictional interludes, and scathing allusions. Its
inflated style can hardly penetrate the fagade, let
alone deflate humanity.
Q. Which of the following best describes how each passage characterizes Hemingway?
Question based on the following passages.
Passage 1 is adapted from an essay written by John Aldridge in 1951. ©1951 by John Aldridge. Passage 2 is adapted from Brom Weber, "Ernest Hemingway's Genteel Bullfight," published in The American Novel and the Nineteen Twenties. ©1971 by Hodder Education.
Passage 1
By the time we were old enough to read
Hemingway, he had become legendary. Like
Lord Byron a century earlier, he had learned
to play himself, his own best hero, with superb
(5) conviction. He was Hemingway of the rugged
outdoor grin and the hairy chest posing beside a
lion he had just shot. He was Tarzan Hemingway,
crouching in the African bush with elephant gun
at ready. He was War Correspondent Hemingway
(10) writing a play in the Hotel Florida in Madrid
while thirty fascist shells crashed through
the roof. Later, he was Task Force Hemingway
swathed in ammunition belts and defending
his post singlehandedly against fierce German
(15) attacks.
But even without the legend, the chest
beating, wisecracking pose that was later to
seem so incredibly absurd, his impact upon us
was tremendous. The feeling he gave us was one
(20) of immense expansiveness, freedom and, at the
same time, absolute stability and control. We
could follow him, imitate his cold detachment,
through all the doubts and fears of adolescence
and come out pure and untouched. The words
(25) he put down seemed to us to have been carved
from the living stone of life. They conveyed
exactly the taste, smell and feel of experience as
it was, as it might possibly be. And so we began
unconsciously to translate our own sensations
(30) into their terms and to impose on everything
we did and felt the particular emotions they
aroused in us.
The Hemingway time was a good time to
be young. We had much then that the war later
(35) forced out of us, something far greater than
Hemingway's strong formative influence.
Later writers who lost or got rid of Hemingway
have been able to find nothing to put in his
place. They have rejected his time as untrue
(40) for them only to fail at finding themselves in their
own time. Others, in their embarrassment at the
hold he once had over them, have not profited
by the lessons he had to teach, and still others
were never touched by him at all. These last are
(45) perhaps the real unfortunates, for they have been
denied access to a powerful tradition.
Passage 2
One wonders why Hemingway's greatest
works now seem unable to evoke the same sense
of a tottering world that in the 1920s established
(50) Ernest Hemingway's reputation. These novels
should be speaking to us. Our social structure
is as shaken, our philosophical despair as great,
our everyday experience as unsatisfying. We have
had more war than Hemingway ever dreamed
(55) of. Our violence—physical, emotional, and
intellectual—is not inferior to that of the 1920s.
Yet Hemingway's great novels no longer seem to
penetrate deeply the surface of existence.
One begins to doubt that they ever did so significantly
(60) in the 1920s.
Hemingway's novels indulged the dominant
genteel tradition in American culture while
seeming to repudiate it. They yielded to the
functionalist, technological aesthetic of the
(65) culture instead of resisting in the manner of
Frank Lloyd Wright. Hemingway, in effect, became a
dupe of his culture rather than its moral-aesthetic
conscience. As a consequence, the import of his
work has diminished. There is some evidence
(70) from his stylistic evolution that Hemingway
himself must have felt as much, for Hemingway's
famous stylistic economy frequently seems to
conceal another kind of writer, with much richer
rhetorical resources to hand. So, Death in the
(75) Afternoon (1932), Hemingway's bullfighting
opus and his first book after A Farewell to Arms
(1929), reveals great uneasiness over his earlier
accomplishment. In it, he defends his literary
method with a doctrine of ambiguity: “If a writer
(80) of prose knows enough about what he is writing
about he may omit things that he knows and
the reader, if the writer is writing truly enough,
will have a feeling of those things as strongly as
though the writer had stated them.”
(85) Hemingway made much the same theoretical
point in another way in Death in the Afternoon
apparently believing that a formal reduction of
aesthetic complexity was the only kind of design
that had value.
(90) Perhaps the greatest irony of Death in the
Afternoon is its unmistakably baroque prose,
which Hemingway himself embarrassedly
admitted was “flowery.” Reviewers, unable to
challenge Hemingway's expertise in the art of
(95) bullfighting, noted that its style was “awkward,
tortuous, [and] belligerently clumsy.”
Death in the Afternoon is an extraordinarily
self-indulgent, unruly, clownish, garrulous,
and satiric book, with scrambled chronologies,
(100) willful digressions, mock-scholarly apparatuses,
fictional interludes, and scathing allusions. Its
inflated style can hardly penetrate the fagade, let
alone deflate humanity.
Q. Which statement about Hemingway is supported by both passages?
Question based on the following passages.
Passage 1 is adapted from an essay written by John Aldridge in 1951. ©1951 by John Aldridge. Passage 2 is adapted from Brom Weber, "Ernest Hemingway's Genteel Bullfight," published in The American Novel and the Nineteen Twenties. ©1971 by Hodder Education.
Passage 1
By the time we were old enough to read
Hemingway, he had become legendary. Like
Lord Byron a century earlier, he had learned
to play himself, his own best hero, with superb
(5) conviction. He was Hemingway of the rugged
outdoor grin and the hairy chest posing beside a
lion he had just shot. He was Tarzan Hemingway,
crouching in the African bush with elephant gun
at ready. He was War Correspondent Hemingway
(10) writing a play in the Hotel Florida in Madrid
while thirty fascist shells crashed through
the roof. Later, he was Task Force Hemingway
swathed in ammunition belts and defending
his post singlehandedly against fierce German
(15) attacks.
But even without the legend, the chest
beating, wisecracking pose that was later to
seem so incredibly absurd, his impact upon us
was tremendous. The feeling he gave us was one
(20) of immense expansiveness, freedom and, at the
same time, absolute stability and control. We
could follow him, imitate his cold detachment,
through all the doubts and fears of adolescence
and come out pure and untouched. The words
(25) he put down seemed to us to have been carved
from the living stone of life. They conveyed
exactly the taste, smell and feel of experience as
it was, as it might possibly be. And so we began
unconsciously to translate our own sensations
(30) into their terms and to impose on everything
we did and felt the particular emotions they
aroused in us.
The Hemingway time was a good time to
be young. We had much then that the war later
(35) forced out of us, something far greater than
Hemingway's strong formative influence.
Later writers who lost or got rid of Hemingway
have been able to find nothing to put in his
place. They have rejected his time as untrue
(40) for them only to fail at finding themselves in their
own time. Others, in their embarrassment at the
hold he once had over them, have not profited
by the lessons he had to teach, and still others
were never touched by him at all. These last are
(45) perhaps the real unfortunates, for they have been
denied access to a powerful tradition.
Passage 2
One wonders why Hemingway's greatest
works now seem unable to evoke the same sense
of a tottering world that in the 1920s established
(50) Ernest Hemingway's reputation. These novels
should be speaking to us. Our social structure
is as shaken, our philosophical despair as great,
our everyday experience as unsatisfying. We have
had more war than Hemingway ever dreamed
(55) of. Our violence—physical, emotional, and
intellectual—is not inferior to that of the 1920s.
Yet Hemingway's great novels no longer seem to
penetrate deeply the surface of existence.
One begins to doubt that they ever did so significantly
(60) in the 1920s.
Hemingway's novels indulged the dominant
genteel tradition in American culture while
seeming to repudiate it. They yielded to the
functionalist, technological aesthetic of the
(65) culture instead of resisting in the manner of
Frank Lloyd Wright. Hemingway, in effect, became a
dupe of his culture rather than its moral-aesthetic
conscience. As a consequence, the import of his
work has diminished. There is some evidence
(70) from his stylistic evolution that Hemingway
himself must have felt as much, for Hemingway's
famous stylistic economy frequently seems to
conceal another kind of writer, with much richer
rhetorical resources to hand. So, Death in the
(75) Afternoon (1932), Hemingway's bullfighting
opus and his first book after A Farewell to Arms
(1929), reveals great uneasiness over his earlier
accomplishment. In it, he defends his literary
method with a doctrine of ambiguity: “If a writer
(80) of prose knows enough about what he is writing
about he may omit things that he knows and
the reader, if the writer is writing truly enough,
will have a feeling of those things as strongly as
though the writer had stated them.”
(85) Hemingway made much the same theoretical
point in another way in Death in the Afternoon
apparently believing that a formal reduction of
aesthetic complexity was the only kind of design
that had value.
(90) Perhaps the greatest irony of Death in the
Afternoon is its unmistakably baroque prose,
which Hemingway himself embarrassedly
admitted was “flowery.” Reviewers, unable to
challenge Hemingway's expertise in the art of
(95) bullfighting, noted that its style was “awkward,
tortuous, [and] belligerently clumsy.”
Death in the Afternoon is an extraordinarily
self-indulgent, unruly, clownish, garrulous,
and satiric book, with scrambled chronologies,
(100) willful digressions, mock-scholarly apparatuses,
fictional interludes, and scathing allusions. Its
inflated style can hardly penetrate the fagade, let
alone deflate humanity.
Q. In line 26, the phrase “living stone” most nearly means
Question based on the following passages.
Passage 1 is adapted from an essay written by John Aldridge in 1951. ©1951 by John Aldridge. Passage 2 is adapted from Brom Weber, "Ernest Hemingway's Genteel Bullfight," published in The American Novel and the Nineteen Twenties. ©1971 by Hodder Education.
Passage 1
By the time we were old enough to read
Hemingway, he had become legendary. Like
Lord Byron a century earlier, he had learned
to play himself, his own best hero, with superb
(5) conviction. He was Hemingway of the rugged
outdoor grin and the hairy chest posing beside a
lion he had just shot. He was Tarzan Hemingway,
crouching in the African bush with elephant gun
at ready. He was War Correspondent Hemingway
(10) writing a play in the Hotel Florida in Madrid
while thirty fascist shells crashed through
the roof. Later, he was Task Force Hemingway
swathed in ammunition belts and defending
his post singlehandedly against fierce German
(15) attacks.
But even without the legend, the chest
beating, wisecracking pose that was later to
seem so incredibly absurd, his impact upon us
was tremendous. The feeling he gave us was one
(20) of immense expansiveness, freedom and, at the
same time, absolute stability and control. We
could follow him, imitate his cold detachment,
through all the doubts and fears of adolescence
and come out pure and untouched. The words
(25) he put down seemed to us to have been carved
from the living stone of life. They conveyed
exactly the taste, smell and feel of experience as
it was, as it might possibly be. And so we began
unconsciously to translate our own sensations
(30) into their terms and to impose on everything
we did and felt the particular emotions they
aroused in us.
The Hemingway time was a good time to
be young. We had much then that the war later
(35) forced out of us, something far greater than
Hemingway's strong formative influence.
Later writers who lost or got rid of Hemingway
have been able to find nothing to put in his
place. They have rejected his time as untrue
(40) for them only to fail at finding themselves in their
own time. Others, in their embarrassment at the
hold he once had over them, have not profited
by the lessons he had to teach, and still others
were never touched by him at all. These last are
(45) perhaps the real unfortunates, for they have been
denied access to a powerful tradition.
Passage 2
One wonders why Hemingway's greatest
works now seem unable to evoke the same sense
of a tottering world that in the 1920s established
(50) Ernest Hemingway's reputation. These novels
should be speaking to us. Our social structure
is as shaken, our philosophical despair as great,
our everyday experience as unsatisfying. We have
had more war than Hemingway ever dreamed
(55) of. Our violence—physical, emotional, and
intellectual—is not inferior to that of the 1920s.
Yet Hemingway's great novels no longer seem to
penetrate deeply the surface of existence.
One begins to doubt that they ever did so significantly
(60) in the 1920s.
Hemingway's novels indulged the dominant
genteel tradition in American culture while
seeming to repudiate it. They yielded to the
functionalist, technological aesthetic of the
(65) culture instead of resisting in the manner of
Frank Lloyd Wright. Hemingway, in effect, became a
dupe of his culture rather than its moral-aesthetic
conscience. As a consequence, the import of his
work has diminished. There is some evidence
(70) from his stylistic evolution that Hemingway
himself must have felt as much, for Hemingway's
famous stylistic economy frequently seems to
conceal another kind of writer, with much richer
rhetorical resources to hand. So, Death in the
(75) Afternoon (1932), Hemingway's bullfighting
opus and his first book after A Farewell to Arms
(1929), reveals great uneasiness over his earlier
accomplishment. In it, he defends his literary
method with a doctrine of ambiguity: “If a writer
(80) of prose knows enough about what he is writing
about he may omit things that he knows and
the reader, if the writer is writing truly enough,
will have a feeling of those things as strongly as
though the writer had stated them.”
(85) Hemingway made much the same theoretical
point in another way in Death in the Afternoon
apparently believing that a formal reduction of
aesthetic complexity was the only kind of design
that had value.
(90) Perhaps the greatest irony of Death in the
Afternoon is its unmistakably baroque prose,
which Hemingway himself embarrassedly
admitted was “flowery.” Reviewers, unable to
challenge Hemingway's expertise in the art of
(95) bullfighting, noted that its style was “awkward,
tortuous, [and] belligerently clumsy.”
Death in the Afternoon is an extraordinarily
self-indulgent, unruly, clownish, garrulous,
and satiric book, with scrambled chronologies,
(100) willful digressions, mock-scholarly apparatuses,
fictional interludes, and scathing allusions. Its
inflated style can hardly penetrate the fagade, let
alone deflate humanity.
Q. Lines 28–32 (“And so we . . . aroused in us”) suggests that many of Hemingway’s readers were inclined to
Question based on the following passages.
Passage 1 is adapted from an essay written by John Aldridge in 1951. ©1951 by John Aldridge. Passage 2 is adapted from Brom Weber, "Ernest Hemingway's Genteel Bullfight," published in The American Novel and the Nineteen Twenties. ©1971 by Hodder Education.
Passage 1
By the time we were old enough to read
Hemingway, he had become legendary. Like
Lord Byron a century earlier, he had learned
to play himself, his own best hero, with superb
(5) conviction. He was Hemingway of the rugged
outdoor grin and the hairy chest posing beside a
lion he had just shot. He was Tarzan Hemingway,
crouching in the African bush with elephant gun
at ready. He was War Correspondent Hemingway
(10) writing a play in the Hotel Florida in Madrid
while thirty fascist shells crashed through
the roof. Later, he was Task Force Hemingway
swathed in ammunition belts and defending
his post singlehandedly against fierce German
(15) attacks.
But even without the legend, the chest
beating, wisecracking pose that was later to
seem so incredibly absurd, his impact upon us
was tremendous. The feeling he gave us was one
(20) of immense expansiveness, freedom and, at the
same time, absolute stability and control. We
could follow him, imitate his cold detachment,
through all the doubts and fears of adolescence
and come out pure and untouched. The words
(25) he put down seemed to us to have been carved
from the living stone of life. They conveyed
exactly the taste, smell and feel of experience as
it was, as it might possibly be. And so we began
unconsciously to translate our own sensations
(30) into their terms and to impose on everything
we did and felt the particular emotions they
aroused in us.
The Hemingway time was a good time to
be young. We had much then that the war later
(35) forced out of us, something far greater than
Hemingway's strong formative influence.
Later writers who lost or got rid of Hemingway
have been able to find nothing to put in his
place. They have rejected his time as untrue
(40) for them only to fail at finding themselves in their
own time. Others, in their embarrassment at the
hold he once had over them, have not profited
by the lessons he had to teach, and still others
were never touched by him at all. These last are
(45) perhaps the real unfortunates, for they have been
denied access to a powerful tradition.
Passage 2
One wonders why Hemingway's greatest
works now seem unable to evoke the same sense
of a tottering world that in the 1920s established
(50) Ernest Hemingway's reputation. These novels
should be speaking to us. Our social structure
is as shaken, our philosophical despair as great,
our everyday experience as unsatisfying. We have
had more war than Hemingway ever dreamed
(55) of. Our violence—physical, emotional, and
intellectual—is not inferior to that of the 1920s.
Yet Hemingway's great novels no longer seem to
penetrate deeply the surface of existence.
One begins to doubt that they ever did so significantly
(60) in the 1920s.
Hemingway's novels indulged the dominant
genteel tradition in American culture while
seeming to repudiate it. They yielded to the
functionalist, technological aesthetic of the
(65) culture instead of resisting in the manner of
Frank Lloyd Wright. Hemingway, in effect, became a
dupe of his culture rather than its moral-aesthetic
conscience. As a consequence, the import of his
work has diminished. There is some evidence
(70) from his stylistic evolution that Hemingway
himself must have felt as much, for Hemingway's
famous stylistic economy frequently seems to
conceal another kind of writer, with much richer
rhetorical resources to hand. So, Death in the
(75) Afternoon (1932), Hemingway's bullfighting
opus and his first book after A Farewell to Arms
(1929), reveals great uneasiness over his earlier
accomplishment. In it, he defends his literary
method with a doctrine of ambiguity: “If a writer
(80) of prose knows enough about what he is writing
about he may omit things that he knows and
the reader, if the writer is writing truly enough,
will have a feeling of those things as strongly as
though the writer had stated them.”
(85) Hemingway made much the same theoretical
point in another way in Death in the Afternoon
apparently believing that a formal reduction of
aesthetic complexity was the only kind of design
that had value.
(90) Perhaps the greatest irony of Death in the
Afternoon is its unmistakably baroque prose,
which Hemingway himself embarrassedly
admitted was “flowery.” Reviewers, unable to
challenge Hemingway's expertise in the art of
(95) bullfighting, noted that its style was “awkward,
tortuous, [and] belligerently clumsy.”
Death in the Afternoon is an extraordinarily
self-indulgent, unruly, clownish, garrulous,
and satiric book, with scrambled chronologies,
(100) willful digressions, mock-scholarly apparatuses,
fictional interludes, and scathing allusions. Its
inflated style can hardly penetrate the fagade, let
alone deflate humanity.
Q. The “lessons” mentioned in line 43 most likely include stories of
Question based on the following passages.
Passage 1 is adapted from an essay written by John Aldridge in 1951. ©1951 by John Aldridge. Passage 2 is adapted from Brom Weber, "Ernest Hemingway's Genteel Bullfight," published in The American Novel and the Nineteen Twenties. ©1971 by Hodder Education.
Passage 1
By the time we were old enough to read
Hemingway, he had become legendary. Like
Lord Byron a century earlier, he had learned
to play himself, his own best hero, with superb
(5) conviction. He was Hemingway of the rugged
outdoor grin and the hairy chest posing beside a
lion he had just shot. He was Tarzan Hemingway,
crouching in the African bush with elephant gun
at ready. He was War Correspondent Hemingway
(10) writing a play in the Hotel Florida in Madrid
while thirty fascist shells crashed through
the roof. Later, he was Task Force Hemingway
swathed in ammunition belts and defending
his post singlehandedly against fierce German
(15) attacks.
But even without the legend, the chest
beating, wisecracking pose that was later to
seem so incredibly absurd, his impact upon us
was tremendous. The feeling he gave us was one
(20) of immense expansiveness, freedom and, at the
same time, absolute stability and control. We
could follow him, imitate his cold detachment,
through all the doubts and fears of adolescence
and come out pure and untouched. The words
(25) he put down seemed to us to have been carved
from the living stone of life. They conveyed
exactly the taste, smell and feel of experience as
it was, as it might possibly be. And so we began
unconsciously to translate our own sensations
(30) into their terms and to impose on everything
we did and felt the particular emotions they
aroused in us.
The Hemingway time was a good time to
be young. We had much then that the war later
(35) forced out of us, something far greater than
Hemingway's strong formative influence.
Later writers who lost or got rid of Hemingway
have been able to find nothing to put in his
place. They have rejected his time as untrue
(40) for them only to fail at finding themselves in their
own time. Others, in their embarrassment at the
hold he once had over them, have not profited
by the lessons he had to teach, and still others
were never touched by him at all. These last are
(45) perhaps the real unfortunates, for they have been
denied access to a powerful tradition.
Passage 2
One wonders why Hemingway's greatest
works now seem unable to evoke the same sense
of a tottering world that in the 1920s established
(50) Ernest Hemingway's reputation. These novels
should be speaking to us. Our social structure
is as shaken, our philosophical despair as great,
our everyday experience as unsatisfying. We have
had more war than Hemingway ever dreamed
(55) of. Our violence—physical, emotional, and
intellectual—is not inferior to that of the 1920s.
Yet Hemingway's great novels no longer seem to
penetrate deeply the surface of existence.
One begins to doubt that they ever did so significantly
(60) in the 1920s.
Hemingway's novels indulged the dominant
genteel tradition in American culture while
seeming to repudiate it. They yielded to the
functionalist, technological aesthetic of the
(65) culture instead of resisting in the manner of
Frank Lloyd Wright. Hemingway, in effect, became a
dupe of his culture rather than its moral-aesthetic
conscience. As a consequence, the import of his
work has diminished. There is some evidence
(70) from his stylistic evolution that Hemingway
himself must have felt as much, for Hemingway's
famous stylistic economy frequently seems to
conceal another kind of writer, with much richer
rhetorical resources to hand. So, Death in the
(75) Afternoon (1932), Hemingway's bullfighting
opus and his first book after A Farewell to Arms
(1929), reveals great uneasiness over his earlier
accomplishment. In it, he defends his literary
method with a doctrine of ambiguity: “If a writer
(80) of prose knows enough about what he is writing
about he may omit things that he knows and
the reader, if the writer is writing truly enough,
will have a feeling of those things as strongly as
though the writer had stated them.”
(85) Hemingway made much the same theoretical
point in another way in Death in the Afternoon
apparently believing that a formal reduction of
aesthetic complexity was the only kind of design
that had value.
(90) Perhaps the greatest irony of Death in the
Afternoon is its unmistakably baroque prose,
which Hemingway himself embarrassedly
admitted was “flowery.” Reviewers, unable to
challenge Hemingway's expertise in the art of
(95) bullfighting, noted that its style was “awkward,
tortuous, [and] belligerently clumsy.”
Death in the Afternoon is an extraordinarily
self-indulgent, unruly, clownish, garrulous,
and satiric book, with scrambled chronologies,
(100) willful digressions, mock-scholarly apparatuses,
fictional interludes, and scathing allusions. Its
inflated style can hardly penetrate the fagade, let
alone deflate humanity.
Q. In line 49, the word “tottering” is intended to evoke a sense of
Question based on the following passages.
Passage 1 is adapted from an essay written by John Aldridge in 1951. ©1951 by John Aldridge. Passage 2 is adapted from Brom Weber, "Ernest Hemingway's Genteel Bullfight," published in The American Novel and the Nineteen Twenties. ©1971 by Hodder Education.
Passage 1
By the time we were old enough to read
Hemingway, he had become legendary. Like
Lord Byron a century earlier, he had learned
to play himself, his own best hero, with superb
(5) conviction. He was Hemingway of the rugged
outdoor grin and the hairy chest posing beside a
lion he had just shot. He was Tarzan Hemingway,
crouching in the African bush with elephant gun
at ready. He was War Correspondent Hemingway
(10) writing a play in the Hotel Florida in Madrid
while thirty fascist shells crashed through
the roof. Later, he was Task Force Hemingway
swathed in ammunition belts and defending
his post singlehandedly against fierce German
(15) attacks.
But even without the legend, the chest
beating, wisecracking pose that was later to
seem so incredibly absurd, his impact upon us
was tremendous. The feeling he gave us was one
(20) of immense expansiveness, freedom and, at the
same time, absolute stability and control. We
could follow him, imitate his cold detachment,
through all the doubts and fears of adolescence
and come out pure and untouched. The words
(25) he put down seemed to us to have been carved
from the living stone of life. They conveyed
exactly the taste, smell and feel of experience as
it was, as it might possibly be. And so we began
unconsciously to translate our own sensations
(30) into their terms and to impose on everything
we did and felt the particular emotions they
aroused in us.
The Hemingway time was a good time to
be young. We had much then that the war later
(35) forced out of us, something far greater than
Hemingway's strong formative influence.
Later writers who lost or got rid of Hemingway
have been able to find nothing to put in his
place. They have rejected his time as untrue
(40) for them only to fail at finding themselves in their
own time. Others, in their embarrassment at the
hold he once had over them, have not profited
by the lessons he had to teach, and still others
were never touched by him at all. These last are
(45) perhaps the real unfortunates, for they have been
denied access to a powerful tradition.
Passage 2
One wonders why Hemingway's greatest
works now seem unable to evoke the same sense
of a tottering world that in the 1920s established
(50) Ernest Hemingway's reputation. These novels
should be speaking to us. Our social structure
is as shaken, our philosophical despair as great,
our everyday experience as unsatisfying. We have
had more war than Hemingway ever dreamed
(55) of. Our violence—physical, emotional, and
intellectual—is not inferior to that of the 1920s.
Yet Hemingway's great novels no longer seem to
penetrate deeply the surface of existence.
One begins to doubt that they ever did so significantly
(60) in the 1920s.
Hemingway's novels indulged the dominant
genteel tradition in American culture while
seeming to repudiate it. They yielded to the
functionalist, technological aesthetic of the
(65) culture instead of resisting in the manner of
Frank Lloyd Wright. Hemingway, in effect, became a
dupe of his culture rather than its moral-aesthetic
conscience. As a consequence, the import of his
work has diminished. There is some evidence
(70) from his stylistic evolution that Hemingway
himself must have felt as much, for Hemingway's
famous stylistic economy frequently seems to
conceal another kind of writer, with much richer
rhetorical resources to hand. So, Death in the
(75) Afternoon (1932), Hemingway's bullfighting
opus and his first book after A Farewell to Arms
(1929), reveals great uneasiness over his earlier
accomplishment. In it, he defends his literary
method with a doctrine of ambiguity: “If a writer
(80) of prose knows enough about what he is writing
about he may omit things that he knows and
the reader, if the writer is writing truly enough,
will have a feeling of those things as strongly as
though the writer had stated them.”
(85) Hemingway made much the same theoretical
point in another way in Death in the Afternoon
apparently believing that a formal reduction of
aesthetic complexity was the only kind of design
that had value.
(90) Perhaps the greatest irony of Death in the
Afternoon is its unmistakably baroque prose,
which Hemingway himself embarrassedly
admitted was “flowery.” Reviewers, unable to
challenge Hemingway's expertise in the art of
(95) bullfighting, noted that its style was “awkward,
tortuous, [and] belligerently clumsy.”
Death in the Afternoon is an extraordinarily
self-indulgent, unruly, clownish, garrulous,
and satiric book, with scrambled chronologies,
(100) willful digressions, mock-scholarly apparatuses,
fictional interludes, and scathing allusions. Its
inflated style can hardly penetrate the fagade, let
alone deflate humanity.
Q. The author of Passage 1 would most likely regard the statement in lines 66–68 (“Hemingway, in effect . . . conscience”), with
Question based on the following passages.
Passage 1 is adapted from an essay written by John Aldridge in 1951. ©1951 by John Aldridge. Passage 2 is adapted from Brom Weber, "Ernest Hemingway's Genteel Bullfight," published in The American Novel and the Nineteen Twenties. ©1971 by Hodder Education.
Passage 1
By the time we were old enough to read
Hemingway, he had become legendary. Like
Lord Byron a century earlier, he had learned
to play himself, his own best hero, with superb
(5) conviction. He was Hemingway of the rugged
outdoor grin and the hairy chest posing beside a
lion he had just shot. He was Tarzan Hemingway,
crouching in the African bush with elephant gun
at ready. He was War Correspondent Hemingway
(10) writing a play in the Hotel Florida in Madrid
while thirty fascist shells crashed through
the roof. Later, he was Task Force Hemingway
swathed in ammunition belts and defending
his post singlehandedly against fierce German
(15) attacks.
But even without the legend, the chest
beating, wisecracking pose that was later to
seem so incredibly absurd, his impact upon us
was tremendous. The feeling he gave us was one
(20) of immense expansiveness, freedom and, at the
same time, absolute stability and control. We
could follow him, imitate his cold detachment,
through all the doubts and fears of adolescence
and come out pure and untouched. The words
(25) he put down seemed to us to have been carved
from the living stone of life. They conveyed
exactly the taste, smell and feel of experience as
it was, as it might possibly be. And so we began
unconsciously to translate our own sensations
(30) into their terms and to impose on everything
we did and felt the particular emotions they
aroused in us.
The Hemingway time was a good time to
be young. We had much then that the war later
(35) forced out of us, something far greater than
Hemingway's strong formative influence.
Later writers who lost or got rid of Hemingway
have been able to find nothing to put in his
place. They have rejected his time as untrue
(40) for them only to fail at finding themselves in their
own time. Others, in their embarrassment at the
hold he once had over them, have not profited
by the lessons he had to teach, and still others
were never touched by him at all. These last are
(45) perhaps the real unfortunates, for they have been
denied access to a powerful tradition.
Passage 2
One wonders why Hemingway's greatest
works now seem unable to evoke the same sense
of a tottering world that in the 1920s established
(50) Ernest Hemingway's reputation. These novels
should be speaking to us. Our social structure
is as shaken, our philosophical despair as great,
our everyday experience as unsatisfying. We have
had more war than Hemingway ever dreamed
(55) of. Our violence—physical, emotional, and
intellectual—is not inferior to that of the 1920s.
Yet Hemingway's great novels no longer seem to
penetrate deeply the surface of existence.
One begins to doubt that they ever did so significantly
(60) in the 1920s.
Hemingway's novels indulged the dominant
genteel tradition in American culture while
seeming to repudiate it. They yielded to the
functionalist, technological aesthetic of the
(65) culture instead of resisting in the manner of
Frank Lloyd Wright. Hemingway, in effect, became a
dupe of his culture rather than its moral-aesthetic
conscience. As a consequence, the import of his
work has diminished. There is some evidence
(70) from his stylistic evolution that Hemingway
himself must have felt as much, for Hemingway's
famous stylistic economy frequently seems to
conceal another kind of writer, with much richer
rhetorical resources to hand. So, Death in the
(75) Afternoon (1932), Hemingway's bullfighting
opus and his first book after A Farewell to Arms
(1929), reveals great uneasiness over his earlier
accomplishment. In it, he defends his literary
method with a doctrine of ambiguity: “If a writer
(80) of prose knows enough about what he is writing
about he may omit things that he knows and
the reader, if the writer is writing truly enough,
will have a feeling of those things as strongly as
though the writer had stated them.”
(85) Hemingway made much the same theoretical
point in another way in Death in the Afternoon
apparently believing that a formal reduction of
aesthetic complexity was the only kind of design
that had value.
(90) Perhaps the greatest irony of Death in the
Afternoon is its unmistakably baroque prose,
which Hemingway himself embarrassedly
admitted was “flowery.” Reviewers, unable to
challenge Hemingway's expertise in the art of
(95) bullfighting, noted that its style was “awkward,
tortuous, [and] belligerently clumsy.”
Death in the Afternoon is an extraordinarily
self-indulgent, unruly, clownish, garrulous,
and satiric book, with scrambled chronologies,
(100) willful digressions, mock-scholarly apparatuses,
fictional interludes, and scathing allusions. Its
inflated style can hardly penetrate the fagade, let
alone deflate humanity.
Q. Which statement provides the best evidence for the answer to the previous question?
Question based on the following passages.
Passage 1 is adapted from an essay written by John Aldridge in 1951. ©1951 by John Aldridge. Passage 2 is adapted from Brom Weber, "Ernest Hemingway's Genteel Bullfight," published in The American Novel and the Nineteen Twenties. ©1971 by Hodder Education.
Passage 1
By the time we were old enough to read
Hemingway, he had become legendary. Like
Lord Byron a century earlier, he had learned
to play himself, his own best hero, with superb
(5) conviction. He was Hemingway of the rugged
outdoor grin and the hairy chest posing beside a
lion he had just shot. He was Tarzan Hemingway,
crouching in the African bush with elephant gun
at ready. He was War Correspondent Hemingway
(10) writing a play in the Hotel Florida in Madrid
while thirty fascist shells crashed through
the roof. Later, he was Task Force Hemingway
swathed in ammunition belts and defending
his post singlehandedly against fierce German
(15) attacks.
But even without the legend, the chest
beating, wisecracking pose that was later to
seem so incredibly absurd, his impact upon us
was tremendous. The feeling he gave us was one
(20) of immense expansiveness, freedom and, at the
same time, absolute stability and control. We
could follow him, imitate his cold detachment,
through all the doubts and fears of adolescence
and come out pure and untouched. The words
(25) he put down seemed to us to have been carved
from the living stone of life. They conveyed
exactly the taste, smell and feel of experience as
it was, as it might possibly be. And so we began
unconsciously to translate our own sensations
(30) into their terms and to impose on everything
we did and felt the particular emotions they
aroused in us.
The Hemingway time was a good time to
be young. We had much then that the war later
(35) forced out of us, something far greater than
Hemingway's strong formative influence.
Later writers who lost or got rid of Hemingway
have been able to find nothing to put in his
place. They have rejected his time as untrue
(40) for them only to fail at finding themselves in their
own time. Others, in their embarrassment at the
hold he once had over them, have not profited
by the lessons he had to teach, and still others
were never touched by him at all. These last are
(45) perhaps the real unfortunates, for they have been
denied access to a powerful tradition.
Passage 2
One wonders why Hemingway's greatest
works now seem unable to evoke the same sense
of a tottering world that in the 1920s established
(50) Ernest Hemingway's reputation. These novels
should be speaking to us. Our social structure
is as shaken, our philosophical despair as great,
our everyday experience as unsatisfying. We have
had more war than Hemingway ever dreamed
(55) of. Our violence—physical, emotional, and
intellectual—is not inferior to that of the 1920s.
Yet Hemingway's great novels no longer seem to
penetrate deeply the surface of existence.
One begins to doubt that they ever did so significantly
(60) in the 1920s.
Hemingway's novels indulged the dominant
genteel tradition in American culture while
seeming to repudiate it. They yielded to the
functionalist, technological aesthetic of the
(65) culture instead of resisting in the manner of
Frank Lloyd Wright. Hemingway, in effect, became a
dupe of his culture rather than its moral-aesthetic
conscience. As a consequence, the import of his
work has diminished. There is some evidence
(70) from his stylistic evolution that Hemingway
himself must have felt as much, for Hemingway's
famous stylistic economy frequently seems to
conceal another kind of writer, with much richer
rhetorical resources to hand. So, Death in the
(75) Afternoon (1932), Hemingway's bullfighting
opus and his first book after A Farewell to Arms
(1929), reveals great uneasiness over his earlier
accomplishment. In it, he defends his literary
method with a doctrine of ambiguity: “If a writer
(80) of prose knows enough about what he is writing
about he may omit things that he knows and
the reader, if the writer is writing truly enough,
will have a feeling of those things as strongly as
though the writer had stated them.”
(85) Hemingway made much the same theoretical
point in another way in Death in the Afternoon
apparently believing that a formal reduction of
aesthetic complexity was the only kind of design
that had value.
(90) Perhaps the greatest irony of Death in the
Afternoon is its unmistakably baroque prose,
which Hemingway himself embarrassedly
admitted was “flowery.” Reviewers, unable to
challenge Hemingway's expertise in the art of
(95) bullfighting, noted that its style was “awkward,
tortuous, [and] belligerently clumsy.”
Death in the Afternoon is an extraordinarily
self-indulgent, unruly, clownish, garrulous,
and satiric book, with scrambled chronologies,
(100) willful digressions, mock-scholarly apparatuses,
fictional interludes, and scathing allusions. Its
inflated style can hardly penetrate the fagade, let
alone deflate humanity.
Q. The author of Passage 2 suggests that, in comparison to Hemingway, Frank Lloyd Wright was relatively
Question based on the following passage.
This passage is from S. K. Mukherjee, “The Mysteries of the Strong Nuclear Force ." ©2015 College Hill Coaching.
As any good contractor will tell you, a sound
structure requires stable materials. But atoms,
the building blocks of everything we know and
love—bunnies, brownies, and best friends—
(5) don't appear to be models of stability. Why are
some atoms, like sodium, so hyperactive while
others, like helium, are so aloof? Why do the
electrons that inhabit atoms jump around so
strangely, from one bizarrely shaped orbital to
(10) another? And why do protons, the bits that
give atoms their heft and personality, stick
together at all?
We are told that every atom has a tiny
nucleus containing positively charged protons
(15) and uncharged neutrons, swarmed by a cloud
of speedy electrons. We are also told that like
charges, such as protons, repel each other with
a force that shoots up to infinity as they get
closer. Even worse, you can't get much closer
(20) than two protons in the nucleus of an atom. So
what's keeping atomic nuclei from flying apart?
Obviously, some other force must be at work
inside the atom, something that we can't detect at
our human scale. Physicists call this the
(25) “strong nuclear force.” But where does it
come from?
In order for this force to account for the
binding of protons in the nucleus, it must have
certain interesting features. First, it can't have any
(30) sizeable effect beyond the radius of the atom itself,
or it would play havoc with the nuclei of adjacent
atoms, destroying matter as we know it. Second,
it must perfectly balance the repulsive force of
electricity at an “equilibrium point” of about
(35) 0.7 x 10-15 meters, the average distance between
bound protons, in order to create a stable nucleus.
Third, it must repel at even shorter distances, or
else neutrons (which don't have any electrostatic
repulsion to balance the strong nuclear force)
(40) would collapse into each other. The graph
shows the behavior of such a force relative
to the repulsive electrostatic force.
In 1935, Japanese physicist Hideki Yukawa
proposed that the nuclear force was conveyed by
(45) a then-undiscovered heavy subatomic particle
he called the pi meson (or “pion”), which (unlike
the photon, which conveys the electrostatic
force) decays very quickly and therefore conveys a
powerful force only over a very short distance.
(50) Professor Yukawa's theory, however, was
dealt a mortal blow by a series of experiments
conducted at Los Alamos National Laboratory
in the early 1990s that demonstrated that pions
carry force only over distances greater than the
(55) distance between bound protons. The pion was a
plumber's wrench trying to do a tweezer's job.
Current atomic theory suggests that the
strong nuclear force is most likely conveyed by
massless particles called “gluons” according
(60) to the theory of quantum chromodynamics, or
QCD for short. According to QCD, protons and
neutrons are composed of smaller particles
called quarks, which are held together by the
aptly named gluons. This quark-binding force has
(65) a “residue” that extends beyond the protons and
neutrons themselves to provide just enough force
to bind the protons and neutrons together.
If you're hoping that QCD ties up atomic
behavior with a tidy little bow, you may be just
(70) a bit disappointed. As a quantum theory, it
conceives of space and time as tiny chunks that
occasionally misbehave, rather than smooth
predictable quantities, and its mathematical
formulas are perhaps as hard to penetrate as the
(75) nucleus itself.
Q. The primary purpose of the first paragraph (lines 1–12) is to
Question based on the following passage.
This passage is from S. K. Mukherjee, “The Mysteries of the Strong Nuclear Force ." ©2015 College Hill Coaching.
As any good contractor will tell you, a sound
structure requires stable materials. But atoms,
the building blocks of everything we know and
love—bunnies, brownies, and best friends—
(5) don't appear to be models of stability. Why are
some atoms, like sodium, so hyperactive while
others, like helium, are so aloof? Why do the
electrons that inhabit atoms jump around so
strangely, from one bizarrely shaped orbital to
(10) another? And why do protons, the bits that
give atoms their heft and personality, stick
together at all?
We are told that every atom has a tiny
nucleus containing positively charged protons
(15) and uncharged neutrons, swarmed by a cloud
of speedy electrons. We are also told that like
charges, such as protons, repel each other with
a force that shoots up to infinity as they get
closer. Even worse, you can't get much closer
(20) than two protons in the nucleus of an atom. So
what's keeping atomic nuclei from flying apart?
Obviously, some other force must be at work
inside the atom, something that we can't detect at
our human scale. Physicists call this the
(25) “strong nuclear force.” But where does it
come from?
In order for this force to account for the
binding of protons in the nucleus, it must have
certain interesting features. First, it can't have any
(30) sizeable effect beyond the radius of the atom itself,
or it would play havoc with the nuclei of adjacent
atoms, destroying matter as we know it. Second,
it must perfectly balance the repulsive force of
electricity at an “equilibrium point” of about
(35) 0.7 x 10-15 meters, the average distance between
bound protons, in order to create a stable nucleus.
Third, it must repel at even shorter distances, or
else neutrons (which don't have any electrostatic
repulsion to balance the strong nuclear force)
(40) would collapse into each other. The graph
shows the behavior of such a force relative
to the repulsive electrostatic force.
In 1935, Japanese physicist Hideki Yukawa
proposed that the nuclear force was conveyed by
(45) a then-undiscovered heavy subatomic particle
he called the pi meson (or “pion”), which (unlike
the photon, which conveys the electrostatic
force) decays very quickly and therefore conveys a
powerful force only over a very short distance.
(50) Professor Yukawa's theory, however, was
dealt a mortal blow by a series of experiments
conducted at Los Alamos National Laboratory
in the early 1990s that demonstrated that pions
carry force only over distances greater than the
(55) distance between bound protons. The pion was a
plumber's wrench trying to do a tweezer's job.
Current atomic theory suggests that the
strong nuclear force is most likely conveyed by
massless particles called “gluons” according
(60) to the theory of quantum chromodynamics, or
QCD for short. According to QCD, protons and
neutrons are composed of smaller particles
called quarks, which are held together by the
aptly named gluons. This quark-binding force has
(65) a “residue” that extends beyond the protons and
neutrons themselves to provide just enough force
to bind the protons and neutrons together.
If you're hoping that QCD ties up atomic
behavior with a tidy little bow, you may be just
(70) a bit disappointed. As a quantum theory, it
conceives of space and time as tiny chunks that
occasionally misbehave, rather than smooth
predictable quantities, and its mathematical
formulas are perhaps as hard to penetrate as the
(75) nucleus itself.
Q. In line 7, “aloof” most nearly means
Question based on the following passage.
This passage is from S. K. Mukherjee, “The Mysteries of the Strong Nuclear Force ." ©2015 College Hill Coaching.
As any good contractor will tell you, a sound
structure requires stable materials. But atoms,
the building blocks of everything we know and
love—bunnies, brownies, and best friends—
(5) don't appear to be models of stability. Why are
some atoms, like sodium, so hyperactive while
others, like helium, are so aloof? Why do the
electrons that inhabit atoms jump around so
strangely, from one bizarrely shaped orbital to
(10) another? And why do protons, the bits that
give atoms their heft and personality, stick
together at all?
We are told that every atom has a tiny
nucleus containing positively charged protons
(15) and uncharged neutrons, swarmed by a cloud
of speedy electrons. We are also told that like
charges, such as protons, repel each other with
a force that shoots up to infinity as they get
closer. Even worse, you can't get much closer
(20) than two protons in the nucleus of an atom. So
what's keeping atomic nuclei from flying apart?
Obviously, some other force must be at work
inside the atom, something that we can't detect at
our human scale. Physicists call this the
(25) “strong nuclear force.” But where does it
come from?
In order for this force to account for the
binding of protons in the nucleus, it must have
certain interesting features. First, it can't have any
(30) sizeable effect beyond the radius of the atom itself,
or it would play havoc with the nuclei of adjacent
atoms, destroying matter as we know it. Second,
it must perfectly balance the repulsive force of
electricity at an “equilibrium point” of about
(35) 0.7 x 10-15 meters, the average distance between
bound protons, in order to create a stable nucleus.
Third, it must repel at even shorter distances, or
else neutrons (which don't have any electrostatic
repulsion to balance the strong nuclear force)
(40) would collapse into each other. The graph
shows the behavior of such a force relative
to the repulsive electrostatic force.
In 1935, Japanese physicist Hideki Yukawa
proposed that the nuclear force was conveyed by
(45) a then-undiscovered heavy subatomic particle
he called the pi meson (or “pion”), which (unlike
the photon, which conveys the electrostatic
force) decays very quickly and therefore conveys a
powerful force only over a very short distance.
(50) Professor Yukawa's theory, however, was
dealt a mortal blow by a series of experiments
conducted at Los Alamos National Laboratory
in the early 1990s that demonstrated that pions
carry force only over distances greater than the
(55) distance between bound protons. The pion was a
plumber's wrench trying to do a tweezer's job.
Current atomic theory suggests that the
strong nuclear force is most likely conveyed by
massless particles called “gluons” according
(60) to the theory of quantum chromodynamics, or
QCD for short. According to QCD, protons and
neutrons are composed of smaller particles
called quarks, which are held together by the
aptly named gluons. This quark-binding force has
(65) a “residue” that extends beyond the protons and
neutrons themselves to provide just enough force
to bind the protons and neutrons together.
If you're hoping that QCD ties up atomic
behavior with a tidy little bow, you may be just
(70) a bit disappointed. As a quantum theory, it
conceives of space and time as tiny chunks that
occasionally misbehave, rather than smooth
predictable quantities, and its mathematical
formulas are perhaps as hard to penetrate as the
(75) nucleus itself.
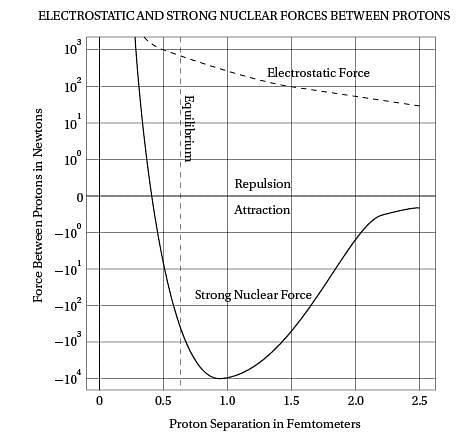
Q. The question in lines 10–12 (“And why . . . at all?”) indicates
Question based on the following passage.
This passage is from S. K. Mukherjee, “The Mysteries of the Strong Nuclear Force ." ©2015 College Hill Coaching.
As any good contractor will tell you, a sound
structure requires stable materials. But atoms,
the building blocks of everything we know and
love—bunnies, brownies, and best friends—
(5) don't appear to be models of stability. Why are
some atoms, like sodium, so hyperactive while
others, like helium, are so aloof? Why do the
electrons that inhabit atoms jump around so
strangely, from one bizarrely shaped orbital to
(10) another? And why do protons, the bits that
give atoms their heft and personality, stick
together at all?
We are told that every atom has a tiny
nucleus containing positively charged protons
(15) and uncharged neutrons, swarmed by a cloud
of speedy electrons. We are also told that like
charges, such as protons, repel each other with
a force that shoots up to infinity as they get
closer. Even worse, you can't get much closer
(20) than two protons in the nucleus of an atom. So
what's keeping atomic nuclei from flying apart?
Obviously, some other force must be at work
inside the atom, something that we can't detect at
our human scale. Physicists call this the
(25) “strong nuclear force.” But where does it
come from?
In order for this force to account for the
binding of protons in the nucleus, it must have
certain interesting features. First, it can't have any
(30) sizeable effect beyond the radius of the atom itself,
or it would play havoc with the nuclei of adjacent
atoms, destroying matter as we know it. Second,
it must perfectly balance the repulsive force of
electricity at an “equilibrium point” of about
(35) 0.7 x 10-15 meters, the average distance between
bound protons, in order to create a stable nucleus.
Third, it must repel at even shorter distances, or
else neutrons (which don't have any electrostatic
repulsion to balance the strong nuclear force)
(40) would collapse into each other. The graph
shows the behavior of such a force relative
to the repulsive electrostatic force.
In 1935, Japanese physicist Hideki Yukawa
proposed that the nuclear force was conveyed by
(45) a then-undiscovered heavy subatomic particle
he called the pi meson (or “pion”), which (unlike
the photon, which conveys the electrostatic
force) decays very quickly and therefore conveys a
powerful force only over a very short distance.
(50) Professor Yukawa's theory, however, was
dealt a mortal blow by a series of experiments
conducted at Los Alamos National Laboratory
in the early 1990s that demonstrated that pions
carry force only over distances greater than the
(55) distance between bound protons. The pion was a
plumber's wrench trying to do a tweezer's job.
Current atomic theory suggests that the
strong nuclear force is most likely conveyed by
massless particles called “gluons” according
(60) to the theory of quantum chromodynamics, or
QCD for short. According to QCD, protons and
neutrons are composed of smaller particles
called quarks, which are held together by the
aptly named gluons. This quark-binding force has
(65) a “residue” that extends beyond the protons and
neutrons themselves to provide just enough force
to bind the protons and neutrons together.
If you're hoping that QCD ties up atomic
behavior with a tidy little bow, you may be just
(70) a bit disappointed. As a quantum theory, it
conceives of space and time as tiny chunks that
occasionally misbehave, rather than smooth
predictable quantities, and its mathematical
formulas are perhaps as hard to penetrate as the
(75) nucleus itself.
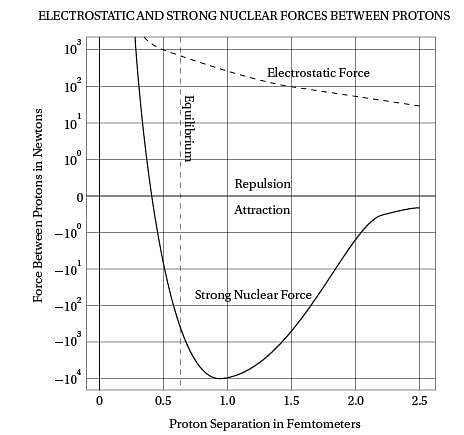
Q. Which sentence provides the best evidence for the answer to the previous question?
Question based on the following passage.
This passage is from S. K. Mukherjee, “The Mysteries of the Strong Nuclear Force ." ©2015 College Hill Coaching.
As any good contractor will tell you, a sound
structure requires stable materials. But atoms,
the building blocks of everything we know and
love—bunnies, brownies, and best friends—
(5) don't appear to be models of stability. Why are
some atoms, like sodium, so hyperactive while
others, like helium, are so aloof? Why do the
electrons that inhabit atoms jump around so
strangely, from one bizarrely shaped orbital to
(10) another? And why do protons, the bits that
give atoms their heft and personality, stick
together at all?
We are told that every atom has a tiny
nucleus containing positively charged protons
(15) and uncharged neutrons, swarmed by a cloud
of speedy electrons. We are also told that like
charges, such as protons, repel each other with
a force that shoots up to infinity as they get
closer. Even worse, you can't get much closer
(20) than two protons in the nucleus of an atom. So
what's keeping atomic nuclei from flying apart?
Obviously, some other force must be at work
inside the atom, something that we can't detect at
our human scale. Physicists call this the
(25) “strong nuclear force.” But where does it
come from?
In order for this force to account for the
binding of protons in the nucleus, it must have
certain interesting features. First, it can't have any
(30) sizeable effect beyond the radius of the atom itself,
or it would play havoc with the nuclei of adjacent
atoms, destroying matter as we know it. Second,
it must perfectly balance the repulsive force of
electricity at an “equilibrium point” of about
(35) 0.7 x 10-15 meters, the average distance between
bound protons, in order to create a stable nucleus.
Third, it must repel at even shorter distances, or
else neutrons (which don't have any electrostatic
repulsion to balance the strong nuclear force)
(40) would collapse into each other. The graph
shows the behavior of such a force relative
to the repulsive electrostatic force.
In 1935, Japanese physicist Hideki Yukawa
proposed that the nuclear force was conveyed by
(45) a then-undiscovered heavy subatomic particle
he called the pi meson (or “pion”), which (unlike
the photon, which conveys the electrostatic
force) decays very quickly and therefore conveys a
powerful force only over a very short distance.
(50) Professor Yukawa's theory, however, was
dealt a mortal blow by a series of experiments
conducted at Los Alamos National Laboratory
in the early 1990s that demonstrated that pions
carry force only over distances greater than the
(55) distance between bound protons. The pion was a
plumber's wrench trying to do a tweezer's job.
Current atomic theory suggests that the
strong nuclear force is most likely conveyed by
massless particles called “gluons” according
(60) to the theory of quantum chromodynamics, or
QCD for short. According to QCD, protons and
neutrons are composed of smaller particles
called quarks, which are held together by the
aptly named gluons. This quark-binding force has
(65) a “residue” that extends beyond the protons and
neutrons themselves to provide just enough force
to bind the protons and neutrons together.
If you're hoping that QCD ties up atomic
behavior with a tidy little bow, you may be just
(70) a bit disappointed. As a quantum theory, it
conceives of space and time as tiny chunks that
occasionally misbehave, rather than smooth
predictable quantities, and its mathematical
formulas are perhaps as hard to penetrate as the
(75) nucleus itself.
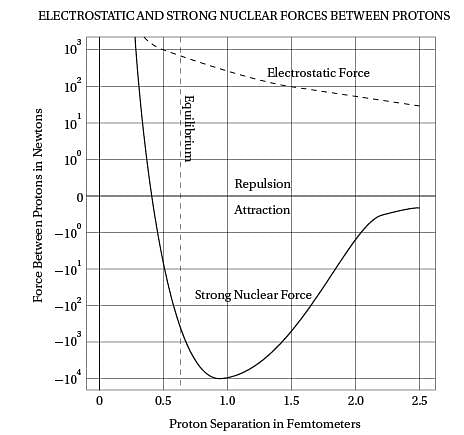
Q. In lines 13–16, the repetition of the phrase “We are” serves primarily to emphasize
Question based on the following passage.
This passage is from S. K. Mukherjee, “The Mysteries of the Strong Nuclear Force ." ©2015 College Hill Coaching.
As any good contractor will tell you, a sound
structure requires stable materials. But atoms,
the building blocks of everything we know and
love—bunnies, brownies, and best friends—
(5) don't appear to be models of stability. Why are
some atoms, like sodium, so hyperactive while
others, like helium, are so aloof? Why do the
electrons that inhabit atoms jump around so
strangely, from one bizarrely shaped orbital to
(10) another? And why do protons, the bits that
give atoms their heft and personality, stick
together at all?
We are told that every atom has a tiny
nucleus containing positively charged protons
(15) and uncharged neutrons, swarmed by a cloud
of speedy electrons. We are also told that like
charges, such as protons, repel each other with
a force that shoots up to infinity as they get
closer. Even worse, you can't get much closer
(20) than two protons in the nucleus of an atom. So
what's keeping atomic nuclei from flying apart?
Obviously, some other force must be at work
inside the atom, something that we can't detect at
our human scale. Physicists call this the
(25) “strong nuclear force.” But where does it
come from?
In order for this force to account for the
binding of protons in the nucleus, it must have
certain interesting features. First, it can't have any
(30) sizeable effect beyond the radius of the atom itself,
or it would play havoc with the nuclei of adjacent
atoms, destroying matter as we know it. Second,
it must perfectly balance the repulsive force of
electricity at an “equilibrium point” of about
(35) 0.7 x 10-15 meters, the average distance between
bound protons, in order to create a stable nucleus.
Third, it must repel at even shorter distances, or
else neutrons (which don't have any electrostatic
repulsion to balance the strong nuclear force)
(40) would collapse into each other. The graph
shows the behavior of such a force relative
to the repulsive electrostatic force.
In 1935, Japanese physicist Hideki Yukawa
proposed that the nuclear force was conveyed by
(45) a then-undiscovered heavy subatomic particle
he called the pi meson (or “pion”), which (unlike
the photon, which conveys the electrostatic
force) decays very quickly and therefore conveys a
powerful force only over a very short distance.
(50) Professor Yukawa's theory, however, was
dealt a mortal blow by a series of experiments
conducted at Los Alamos National Laboratory
in the early 1990s that demonstrated that pions
carry force only over distances greater than the
(55) distance between bound protons. The pion was a
plumber's wrench trying to do a tweezer's job.
Current atomic theory suggests that the
strong nuclear force is most likely conveyed by
massless particles called “gluons” according
(60) to the theory of quantum chromodynamics, or
QCD for short. According to QCD, protons and
neutrons are composed of smaller particles
called quarks, which are held together by the
aptly named gluons. This quark-binding force has
(65) a “residue” that extends beyond the protons and
neutrons themselves to provide just enough force
to bind the protons and neutrons together.
If you're hoping that QCD ties up atomic
behavior with a tidy little bow, you may be just
(70) a bit disappointed. As a quantum theory, it
conceives of space and time as tiny chunks that
occasionally misbehave, rather than smooth
predictable quantities, and its mathematical
formulas are perhaps as hard to penetrate as the
(75) nucleus itself.
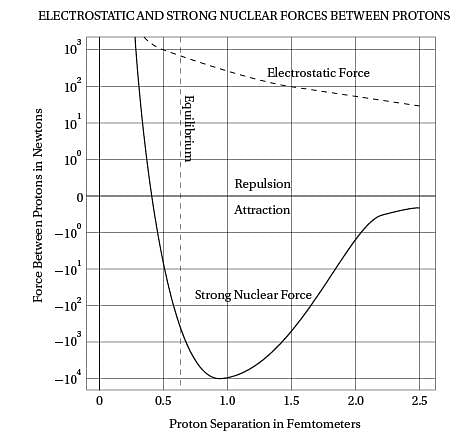
Q. Which of the following best describes the relationship between the electrostatic force and the strong nuclear force between protons at the equilibrium point as shown in the graph?
Question based on the following passage.
This passage is from S. K. Mukherjee, “The Mysteries of the Strong Nuclear Force ." ©2015 College Hill Coaching.
As any good contractor will tell you, a sound
structure requires stable materials. But atoms,
the building blocks of everything we know and
love—bunnies, brownies, and best friends—
(5) don't appear to be models of stability. Why are
some atoms, like sodium, so hyperactive while
others, like helium, are so aloof? Why do the
electrons that inhabit atoms jump around so
strangely, from one bizarrely shaped orbital to
(10) another? And why do protons, the bits that
give atoms their heft and personality, stick
together at all?
We are told that every atom has a tiny
nucleus containing positively charged protons
(15) and uncharged neutrons, swarmed by a cloud
of speedy electrons. We are also told that like
charges, such as protons, repel each other with
a force that shoots up to infinity as they get
closer. Even worse, you can't get much closer
(20) than two protons in the nucleus of an atom. So
what's keeping atomic nuclei from flying apart?
Obviously, some other force must be at work
inside the atom, something that we can't detect at
our human scale. Physicists call this the
(25) “strong nuclear force.” But where does it
come from?
In order for this force to account for the
binding of protons in the nucleus, it must have
certain interesting features. First, it can't have any
(30) sizeable effect beyond the radius of the atom itself,
or it would play havoc with the nuclei of adjacent
atoms, destroying matter as we know it. Second,
it must perfectly balance the repulsive force of
electricity at an “equilibrium point” of about
(35) 0.7 x 10-15 meters, the average distance between
bound protons, in order to create a stable nucleus.
Third, it must repel at even shorter distances, or
else neutrons (which don't have any electrostatic
repulsion to balance the strong nuclear force)
(40) would collapse into each other. The graph
shows the behavior of such a force relative
to the repulsive electrostatic force.
In 1935, Japanese physicist Hideki Yukawa
proposed that the nuclear force was conveyed by
(45) a then-undiscovered heavy subatomic particle
he called the pi meson (or “pion”), which (unlike
the photon, which conveys the electrostatic
force) decays very quickly and therefore conveys a
powerful force only over a very short distance.
(50) Professor Yukawa's theory, however, was
dealt a mortal blow by a series of experiments
conducted at Los Alamos National Laboratory
in the early 1990s that demonstrated that pions
carry force only over distances greater than the
(55) distance between bound protons. The pion was a
plumber's wrench trying to do a tweezer's job.
Current atomic theory suggests that the
strong nuclear force is most likely conveyed by
massless particles called “gluons” according
(60) to the theory of quantum chromodynamics, or
QCD for short. According to QCD, protons and
neutrons are composed of smaller particles
called quarks, which are held together by the
aptly named gluons. This quark-binding force has
(65) a “residue” that extends beyond the protons and
neutrons themselves to provide just enough force
to bind the protons and neutrons together.
If you're hoping that QCD ties up atomic
behavior with a tidy little bow, you may be just
(70) a bit disappointed. As a quantum theory, it
conceives of space and time as tiny chunks that
occasionally misbehave, rather than smooth
predictable quantities, and its mathematical
formulas are perhaps as hard to penetrate as the
(75) nucleus itself.
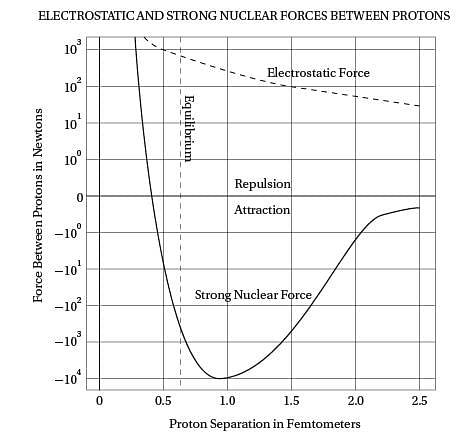
Q. According to the graph, the electrostatic repulsion between two protons separated by 1.5 femtometers is closest to
Question based on the following passage.
This passage is from S. K. Mukherjee, “The Mysteries of the Strong Nuclear Force ." ©2015 College Hill Coaching.
As any good contractor will tell you, a sound
structure requires stable materials. But atoms,
the building blocks of everything we know and
love—bunnies, brownies, and best friends—
(5) don't appear to be models of stability. Why are
some atoms, like sodium, so hyperactive while
others, like helium, are so aloof? Why do the
electrons that inhabit atoms jump around so
strangely, from one bizarrely shaped orbital to
(10) another? And why do protons, the bits that
give atoms their heft and personality, stick
together at all?
We are told that every atom has a tiny
nucleus containing positively charged protons
(15) and uncharged neutrons, swarmed by a cloud
of speedy electrons. We are also told that like
charges, such as protons, repel each other with
a force that shoots up to infinity as they get
closer. Even worse, you can't get much closer
(20) than two protons in the nucleus of an atom. So
what's keeping atomic nuclei from flying apart?
Obviously, some other force must be at work
inside the atom, something that we can't detect at
our human scale. Physicists call this the
(25) “strong nuclear force.” But where does it
come from?
In order for this force to account for the
binding of protons in the nucleus, it must have
certain interesting features. First, it can't have any
(30) sizeable effect beyond the radius of the atom itself,
or it would play havoc with the nuclei of adjacent
atoms, destroying matter as we know it. Second,
it must perfectly balance the repulsive force of
electricity at an “equilibrium point” of about
(35) 0.7 x 10-15 meters, the average distance between
bound protons, in order to create a stable nucleus.
Third, it must repel at even shorter distances, or
else neutrons (which don't have any electrostatic
repulsion to balance the strong nuclear force)
(40) would collapse into each other. The graph
shows the behavior of such a force relative
to the repulsive electrostatic force.
In 1935, Japanese physicist Hideki Yukawa
proposed that the nuclear force was conveyed by
(45) a then-undiscovered heavy subatomic particle
he called the pi meson (or “pion”), which (unlike
the photon, which conveys the electrostatic
force) decays very quickly and therefore conveys a
powerful force only over a very short distance.
(50) Professor Yukawa's theory, however, was
dealt a mortal blow by a series of experiments
conducted at Los Alamos National Laboratory
in the early 1990s that demonstrated that pions
carry force only over distances greater than the
(55) distance between bound protons. The pion was a
plumber's wrench trying to do a tweezer's job.
Current atomic theory suggests that the
strong nuclear force is most likely conveyed by
massless particles called “gluons” according
(60) to the theory of quantum chromodynamics, or
QCD for short. According to QCD, protons and
neutrons are composed of smaller particles
called quarks, which are held together by the
aptly named gluons. This quark-binding force has
(65) a “residue” that extends beyond the protons and
neutrons themselves to provide just enough force
to bind the protons and neutrons together.
If you're hoping that QCD ties up atomic
behavior with a tidy little bow, you may be just
(70) a bit disappointed. As a quantum theory, it
conceives of space and time as tiny chunks that
occasionally misbehave, rather than smooth
predictable quantities, and its mathematical
formulas are perhaps as hard to penetrate as the
(75) nucleus itself.
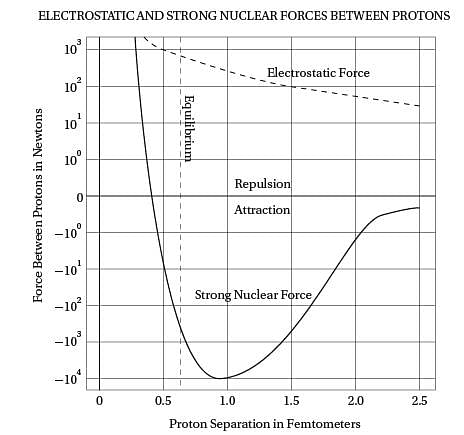
Q. The “mortal blow” (line 51) to Hideki Yukawa’s theory was the fact that
Question based on the following passage.
This passage is from S. K. Mukherjee, “The Mysteries of the Strong Nuclear Force ." ©2015 College Hill Coaching.
As any good contractor will tell you, a sound
structure requires stable materials. But atoms,
the building blocks of everything we know and
love—bunnies, brownies, and best friends—
(5) don't appear to be models of stability. Why are
some atoms, like sodium, so hyperactive while
others, like helium, are so aloof? Why do the
electrons that inhabit atoms jump around so
strangely, from one bizarrely shaped orbital to
(10) another? And why do protons, the bits that
give atoms their heft and personality, stick
together at all?
We are told that every atom has a tiny
nucleus containing positively charged protons
(15) and uncharged neutrons, swarmed by a cloud
of speedy electrons. We are also told that like
charges, such as protons, repel each other with
a force that shoots up to infinity as they get
closer. Even worse, you can't get much closer
(20) than two protons in the nucleus of an atom. So
what's keeping atomic nuclei from flying apart?
Obviously, some other force must be at work
inside the atom, something that we can't detect at
our human scale. Physicists call this the
(25) “strong nuclear force.” But where does it
come from?
In order for this force to account for the
binding of protons in the nucleus, it must have
certain interesting features. First, it can't have any
(30) sizeable effect beyond the radius of the atom itself,
or it would play havoc with the nuclei of adjacent
atoms, destroying matter as we know it. Second,
it must perfectly balance the repulsive force of
electricity at an “equilibrium point” of about
(35) 0.7 x 10-15 meters, the average distance between
bound protons, in order to create a stable nucleus.
Third, it must repel at even shorter distances, or
else neutrons (which don't have any electrostatic
repulsion to balance the strong nuclear force)
(40) would collapse into each other. The graph
shows the behavior of such a force relative
to the repulsive electrostatic force.
In 1935, Japanese physicist Hideki Yukawa
proposed that the nuclear force was conveyed by
(45) a then-undiscovered heavy subatomic particle
he called the pi meson (or “pion”), which (unlike
the photon, which conveys the electrostatic
force) decays very quickly and therefore conveys a
powerful force only over a very short distance.
(50) Professor Yukawa's theory, however, was
dealt a mortal blow by a series of experiments
conducted at Los Alamos National Laboratory
in the early 1990s that demonstrated that pions
carry force only over distances greater than the
(55) distance between bound protons. The pion was a
plumber's wrench trying to do a tweezer's job.
Current atomic theory suggests that the
strong nuclear force is most likely conveyed by
massless particles called “gluons” according
(60) to the theory of quantum chromodynamics, or
QCD for short. According to QCD, protons and
neutrons are composed of smaller particles
called quarks, which are held together by the
aptly named gluons. This quark-binding force has
(65) a “residue” that extends beyond the protons and
neutrons themselves to provide just enough force
to bind the protons and neutrons together.
If you're hoping that QCD ties up atomic
behavior with a tidy little bow, you may be just
(70) a bit disappointed. As a quantum theory, it
conceives of space and time as tiny chunks that
occasionally misbehave, rather than smooth
predictable quantities, and its mathematical
formulas are perhaps as hard to penetrate as the
(75) nucleus itself.
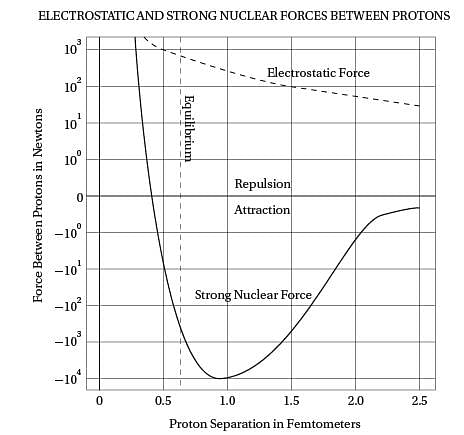
Q. Which of the following best describes the structure of the passage as a whole?
|
8 docs|22 tests
|












