Lakhmir Singh & Manjit Kaur Test: Is Matter Around Us Pure - Class 9 MCQ
25 Questions MCQ Test Science Class 9 - Lakhmir Singh & Manjit Kaur Test: Is Matter Around Us Pure
A combination of common salt and iron filings is a
4 g of solute are dissolved in 36 g of water. What is the mass percent of the solution?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Air shows the property of
At room temperature, a non-metal which is liquid is
Statement A: The separation of constituents from homogeneous mixture is easier as compared to heterogeneous mixture.
Statement B: Milk can be regarded as a pure substance Which of the two statements is correct?
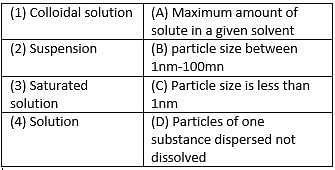
Match the following with correct response.
Statement A: Brass is a solution while gun powder is not
Statement B: Air represents a solution in terms of science
Which of the two statements is true
The element which is a liquid slightly above 300C is
Identify homogeneous mixture from the following
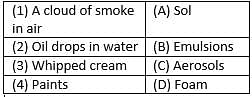
Match the following with correct response.
Find the incorrect statement
Which one is NOT an element?
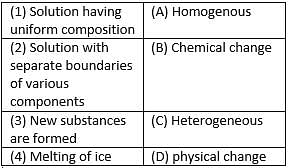
Match the following with correct response.
Diamond is lustrous because
Which one is physical change?
A hard substance when bent produces a tinkling sound. Predict its nature.
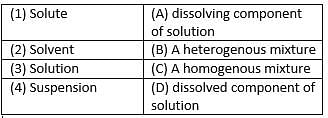
Match the following with the correct response.
1 carat of diamond is equal to
Which of the following are chemical changes?
A shining thick liquid is often used in glass thermometers. Name it.
Cod-liver oil is an example of
How will you bring about the following separation Alcohol from water?
What happens on adding dilute HCl to a mixture of iron filling and sulphur powder?
A. H2S is formed
B. A colour less and odourless gas is formed
C. A greenish solution appears
D. FeS is formed
Thermometer is an instrument that measuring
|
87 videos|369 docs|67 tests
|

















