Test: Bohr’s Model of Atom - NEET MCQ
10 Questions MCQ Test Physics Class 12 - Test: Bohr’s Model of Atom
If the electron in H atom jumps from the third orbit to second orbit, the wavelength of the emitted radiation is given by
The ratio of the speed of the electron in the ground state of hydrogen atom to the speed of light is
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
In hydrogen atom the kinetic energy of electron in an orbit of radius r is given by
In hydrogen atom the angular momentum of the electron in the lowest energy state is
According to Bohr model of hydrogen atom, the radius of stationary orbit characterized by the principal quantum number n is proportional to
Select an incorrect alternative:
i. the radius of the nth orbit is proprtional to n2
ii. the total energy of the electron in the nth orbit is inversely proportional to n
iii. the angular momentum of the electron in nth orbit is an integral multiple of h/2π
iv. the magnitude of potential energy of the electron in any orbit is greater than its kinetic energy
In Bohr model of hydrogen atom, radiation is emitted when the electron
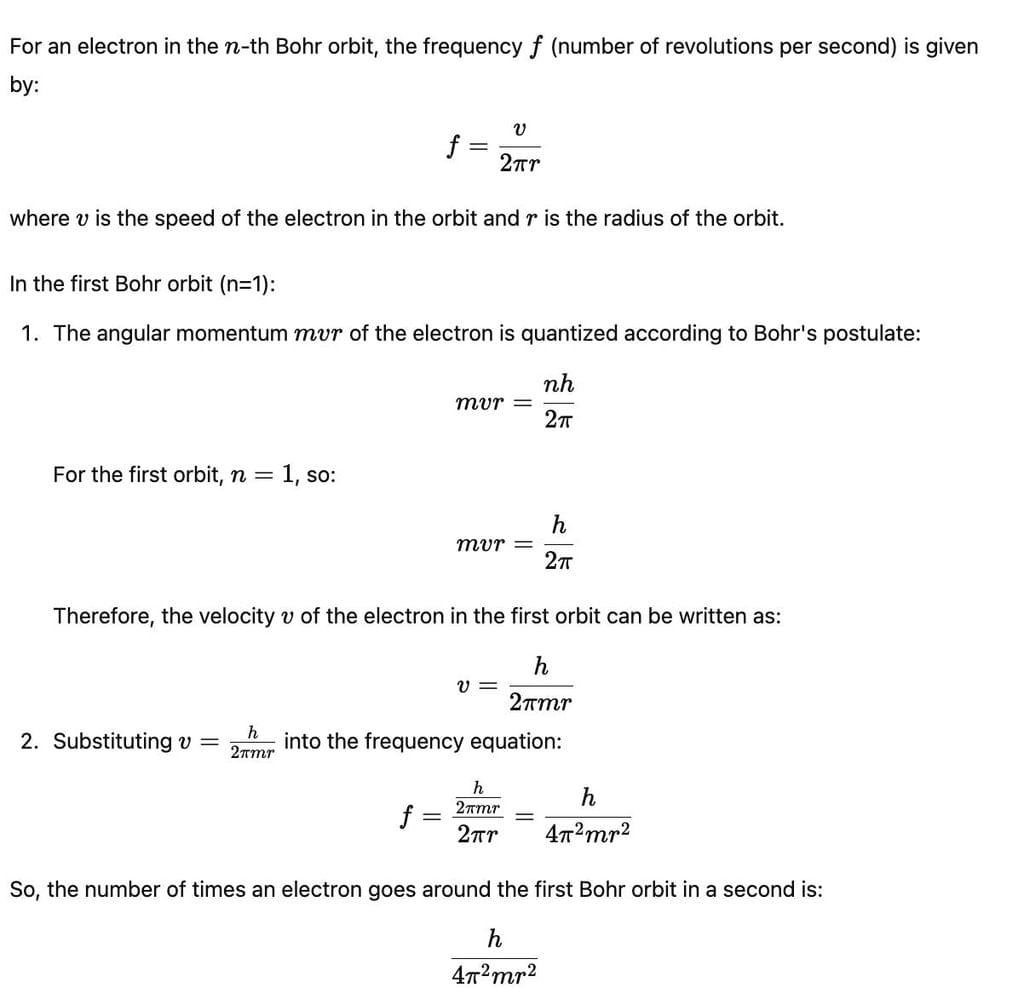
The number of times an electron goes around the first Bohr orbit in a second is
|
105 videos|425 docs|114 tests
|