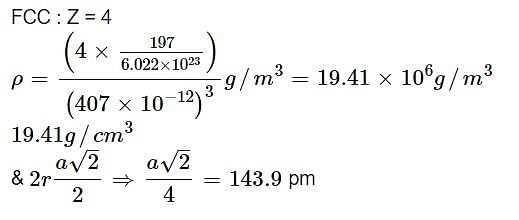Test: Packing Crystals (Old NCERT) - NEET MCQ
22 Questions MCQ Test Chemistry Class 12 - Test: Packing Crystals (Old NCERT)
Only One Option Correct Type
This section contains 11 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q.
CsCI crystallises in a cube that has CP at each corner and Cs+ at the centre of the unit cell. If rCs+ = 169 pm and rcl- =181 pm, then edge length of the cube is
This section contains 11 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
A mineral having the formula AB2 crystallises in the cubic closest - packed lattice with the A-atoms occupying the lattice points. Fraction of the tetrahedral sites occupied by 6-atoms is
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Silver metal crystallises in a cubic closest packed arrangement with edge length 407 pm. Thus, radius of the silver atom is
What is the formula of a magnetic oxide of cobalt used in recording tapes that crystallises with cobalt atoms occupying one-eighth of the tetrahedral holes and one-half of the octahedral holes in a closest packed array of oxide ions?
The total number of tetrahedral voids in the face-centred unit cell is
Packing efficiency (%) of different types of unit cells is given. Select the correct packing efficiency.
Coordination numbers in a square close packed structure and hexagonal close packed structure respectively, are
The length of the unit cell edge of a bcc lattice metal is 352 pm. Thus, volume of atoms in one mole of the metal is
A compound Mp Xq has cubic close packing arrangement of X. Its unit cell structure is shown below. The empirical formula of the compound is
Radii of A+ and that of X- and Y- have been given as
A+= 1.00 pm
X- = 1.00 pm
Y- = 2.00 pm
Thus, ratio of volumes of A X and A Y unit cells is
In the structure of NaCI given below, ratio rNa+/rcl- is
Matching List Type
Choices for the correct combination of elements from Column I and Column II are given as options (a), (b), (c) and (d), out of which one is correct.
One or More than One Options Correct Type
This section contains 5 multiple type questions. Each question has 4 choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Q.
In hexagonal close packing,
Which of the following are correct about the voids formed in three-dimensional hexagonal close-packed structure?
in which of the following arrangements octahedral voids are formed?
Comprehension Type
This section contains a passage describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer out of the given 4 options (a), (b), (c) and (d).
Potassium crystallises in a bcc lattice as shown
Q.
Distance between the two nearest neighbours and between next nearest neighbour respectively, is
Number of neighbours and next - nearest neighbours of K respectively, are
Gold crystallizes in a face centered cubic lattice. If the length of the edge of the unit cell is 407 pm, calculate the density of gold as well as its atomic radius assuming it to be spherical. Atomic mass of gold = 197 amu.
One Integer Value Correct Type
This section contains 3 questions, when worked out will result in an integer value from 0 to 9 (both inclusive).
Atoms of an element B form hep lattice and those o f the element A occupy 2/3rd of tetrahedral voids. How many num ber of atoms of A and B (in total) are present in the hep lattice?
How many octahedral voids are formed in one unit cell of a hep or ccp lattice?
Titanium metal has a density of 4.54 g cm-3 and an edge length o f 412.6 pm. How many atoms are there in the unit cell? (Ti = 48)
|
108 videos|286 docs|123 tests
|