Test: Carboxylic Acid Derivatives - MCAT MCQ
10 Questions MCQ Test Organic Chemistry for MCAT - Test: Carboxylic Acid Derivatives
Which of the following would be the best method of producing methyl propanoate?
Which of the following undergoes a Fischer esterification most rapidly?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which of the following would be most reactive toward nucleophiles?
Which of the following would react most readily with a carboxylic acid to form an amide?
β-lactams are:
I. cyclic forms of the least reactive type of carboxylic acid derivative.
II. more reactive than their straight-chain counterparts.
III. molecules with high levels of ring strain.
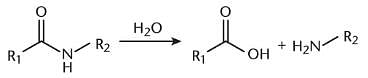
The reaction shown, which is important to breakdown of polypeptides, would be favored under what conditions?
The molecule shown is:
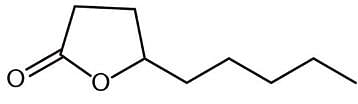
I. synthesizable from a γ-hydroxycarboxylic acid.
II. a lactone.
III. a form of an ester.
Why should esterification reactions NOT be carried out in water?
If propanamide were treated with water, what product(s) would be observed?
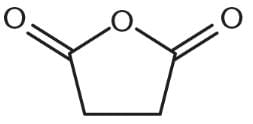
How might succinic anhydride, shown below, be formed from succinic acid (butanedioic acid)?
|
140 videos|5 docs|15 tests
|
|
140 videos|5 docs|15 tests
|

















