Test: Unit Cells, Lattices & Bragg's Equation (Old NCERT) - NEET MCQ
20 Questions MCQ Test Chemistry Class 12 - Test: Unit Cells, Lattices & Bragg's Equation (Old NCERT)
Only One Option Correct Type
This section contains 9 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q. Bravais three-dimensional lattices are of
This section contains 9 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q. Bravais three-dimensional lattices are of
The lattice site in a pure crystal cannot be occupied by
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Number of formula units in unit cells of MgO (rock salt), ZnS (zinc blende) and Pt (fee) respectively are
A compound is formed by elements A and B and has cubic structure. A-atoms are at the corners and B-atoms are at the face centres. Thus, it is
The mass of a unit cell of NaCL corresponds to
At what angles for the first-order diffraction, spacing between two planes X respectively are λ and λ/2 ?
A solid has a structure in which W-atoms are located at the corners of a cubic lattice, OF-atoms at the centre of edges and Na atom at the centre of the cube. The formula for the compound is
The number of atoms in primitive cubic unit cell, body-centred cubic unit cell and face-centred cubic unit cell are A , B and C respectively. Select the correct values.
For a second order diffraction using X-ray B ragg’s equation, for a crystal with separation between two layers as d , following graphical study was observed when varying 0 at different λ.
What is the value of d?
One or More than One Options Correct Type
This section contains 3 multiple type questions. Each question has 4 choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
In which of the following primitive cells axial distances or edge lengths (a, b, c)are different?
Minimum interplanar spacing required for Bragg’s diffraction is:
Which of the following Bravais lattices exist as face centered unit cell?
Comprehension Type
This section contains a passage describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer out of the given 4 options (a), (b), (c) and (d).
Perovskite (A), a mineral containing calcium (o), oxygen and titanium
crystallises in the following cubic unit cell:
Q.
What is the Formula of A?
Perovskite (A), a mineral containing calcium (o), oxygen and titanium
crystallises in the following cubic unit cell:
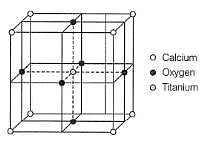
Q. What is the oxidation number of titanium in the given lattice?
Matching List Type
Choices for the correct combination of elements from Column I and Column II are given as options (a), (b), (c) and (d), out of which one is correct.
Match the primitive unit cells given in Column I with their axial angles axial distances given in Column II and select the correct answer from the codes given
One Integer Value Correct Type
This section contains 5 questions, when worked out will result in an integer value from 0 to 9 (both inclusive)
Q. A unit cell is characterised by how many parameters?
How many types of primitive unit cells are there?
In which of the following Bravais lattices, not all axial angles are right angles?
A second order Bragg’s diffraction of X-rays from a set of parallel planes separated by 1.155 occurs at an angle 60° using wavelength of x
. What is the value of x?
Number of unit cells in 240 g of element X (atomic mass 40) which crystallises in bcc pattern in yN 0. What is the value of y?
|
101 videos|287 docs|123 tests
|





















