Test: Oxidation Reduction & Disproportionation Reactions - NEET MCQ
23 Questions MCQ Test Chemistry Class 11 - Test: Oxidation Reduction & Disproportionation Reactions
Direction (Q. Nos. 1-18) This section contains 18 multiple choice questions. Each question has four
choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. Oxidation can be defined as the terms
I. gain of electron and hydrogen
II. gain of oxygen and loss of electron
III. increase in oxidation number
IV. decrease in oxidation number
Select the correct terms
choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
II. gain of oxygen and loss of electron
III. increase in oxidation number
IV. decrease in oxidation number
Reduction is defined in terms of
I. electronation and hydrogenation
II. deelectronation and gain of oxygen
III. increase in oxidation number
IV. decrease in oxidation number
Select the correct terms
II. deelectronation and gain of oxygen
III. increase in oxidation number
IV. decrease in oxidation number
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
In balancing the half-reaction, CN- → CNO-
The number of electrons that must be added is
Select the set of compounds with oxidation-reduction duality.
In the following balanced reaction,
I− reduces IO3- and I2 and itself oxidised to I2 in acidic medium. Thus, final reaction is
Consider the following reactions,
I. Zn + dil. H2SO4 → ZnSO4 + H2
II. Zn + conc. H2SO4 → ZnSO4+ SO2 + H2O
Oxidising agents in I and II are
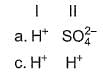
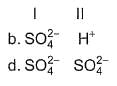
In the following reactions except in one, oxygen is the reducing agent. Exceptional reaction is
In the following reaction,
Which ions are not balanced?
Coefficient x, y and z are respectively
Which of the following species does not show disproportionation reaction?
Which is the intramolecular oxidation-reduction reaction?
Consider the following experimental facts,
I. When Cl2 gas is passed into Kl solution containing CHCI3, violet colour appears in CHCI3 layer.
II. When Cl2 gas is passed into KBr solution containing CHCI3, orange colour appears in CHCI3 layer.
III. When Cl2 gas is passed into a solution containing KBr, Kl and KCI, containing CHCI3, violet colour appears in CHCI3 layer.
Select the correct experimental facts.
Based on the following reaction,
It can be concluded that
For the redox reaction,
x, y and z are
In the following conversion of chromite into soluble chromium salt
4Fe(CrO2)2+ 8Na2CO3 + 7O2 --> 2Fe2O3 + 8Na2CrO4 + 8CO2
There is
In which of the following reactions H2O2 acts as a reducing agent?
Consider the following reaction,
Following reaction, is an example of
Select the correct statement(s).
For the reaction,
The correct statement(s) in the balanced equation is/are
[JEE Advanced 2014]
Identify intramolecular oxidation-reduction reaction.
|
127 videos|244 docs|87 tests
|