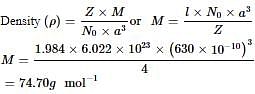Test: The Solid State (Old NCERT) - NEET MCQ
25 Questions MCQ Test Chemistry Class 12 - Test: The Solid State (Old NCERT)
Substances that are strongly attracted by applied magnetic field and can be permanently magnetized are
Which type of solid conduct electricity in molten state but not in solid state?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Yellow colour of NaCl crystals in sodium vapour is due to
Solid X is a very hard electrical insulator in solid as well as in molten state. It melts at extremely high temperature. Solid X is a
Nature of binding forces present in carbondioxide molecules in solid state are?
What type of interaction hold the molecules together in a polar molecular solid?
An atom located at the body center of a cubic unit cell is shared by
The coordination number of AB having rock salt geometry is
MgO has the structure of NaCl. The coordination number of the ions in MgO is
A metal crystallizes with a face-centered cubic lattice. The edge of the unit cell is 408 pm. The diameter of the metal atom is
An element forms a cubic unit cell with edge length 405 pm. Molar mass of this element is 2.7 X 10-2 Kg/mol and its density is given as 2.7 X 103 Kg/m3. How many atoms of this elements are present per unit cell.
A group of 14 element is converted into n – type semiconductor by dopping it with
Group 14 element is converted to p – type semiconductor by dopping it with
A substance forms face centered cubic crystals. Its density is 1.984 g/cm3 and the length of the edge of the unit cell is 630 pm. Calculate the molar mass in g/mol?
In n – type semiconductor current is carried by
Which transition metal oxide has appearance and conductivity like that of copper?
|
108 videos|286 docs|123 tests
|