Chemistry: Topic-wise Test- 2 - NEET MCQ
30 Questions MCQ Test NEET Mock Test Series 2025 - Chemistry: Topic-wise Test- 2
Compound with maximum ionic character is formed from :
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which of the following has a geometry different from the other three species (having the same geometry)?
Which of the following molecule does not have open book structure.
The hybridisation and geometry of BrF3 molecules are :
Which of the following species is not a pseudohalide ?
An orange solid (X) on heating , gives a colourless gas (Y) and a only green residue (Z). Gas (Y) treatment with Mg, produces a white solid substance...............
An inorganic white crystalline compound (A) has a rock salt structure (A) on reaction with conc. H2SO4 and MnO2, evolves a pungent smelling, greenish-yellow gas (B). Compound (A) gives white ppt. of (C) with AgNO3 solution. Compounds (A), (B) and (C) will be respectively
The elements of group 14 are slightly more electronegative than group 13 elements because of
Among the following which is the strongest oxidizing agent?
In the manufacture of sulphuric acid by contact process Tyndall box is used to
If x1, x2 and x3 are enthalpies of H - H, O = O and O - H bonds respectively, and x4 is the enthalpy of vaporisation of water, estimate the standard enthalpy of combustion of hydrogen
The net heat change in a chemical reaction is same whether it is brought about in two or more different ways in one or several steps. It is known as -
According to Hess's Law the thermal effect of a reaction depends on -
Which of the following statem ents regarding free radical halogenation of alkane is not true?
Arrange the following in increasing order of boiling points.
I. 3 -methyl pentane
II. 3-chloropentane
III. 3-bromopentane
IV. 3,3-dichloropentane
What is/are true regarding free radical iodination of an alkane?
Consider the following reaction.
The major product is
Direction (Q. Nos. 8 -11) This section contains 4 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Q. What is /are the expected product in the reaction ?
What are the expected product(s) in the following reaction?
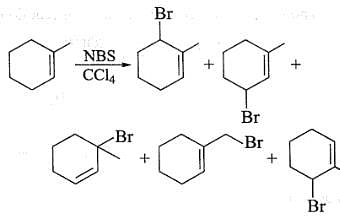
What products are expected in the following reaction?
Terminal alkyne being therm odynamically less stable than an internal alkyne, instead when 2,2-dibromo butane is treated with NaNH2,1-butyne is formed as major product, not 2-butyne because
Statement I : Treatment of either 1,2-dibromobutane or 2,2-dibromobutane with NaNH2 gives the same 1-butyne.
Statement II : NaNH2 forms salt with terminal alkyne which makes possible the above observation.
Statement I : Propyl lithium on reaction with 1-bromo-1-pentyne gives 4-octyne.
Statement II : Propyl lithium acts as a very strong nucleophile, brings about SN2 reaction of halides.
Statement l : C6H5MgBr with propyne gives 1-phenyl propyne.
|
1 videos|26 docs|111 tests
|
|
1 videos|26 docs|111 tests
|