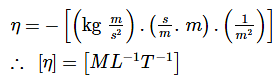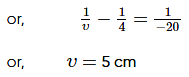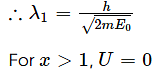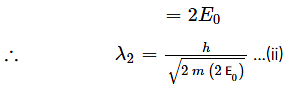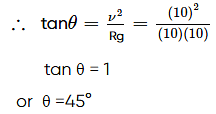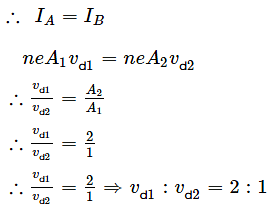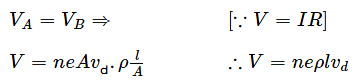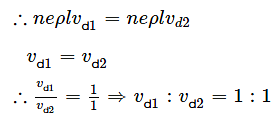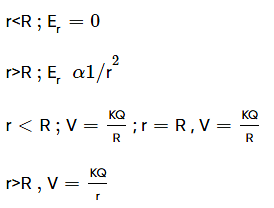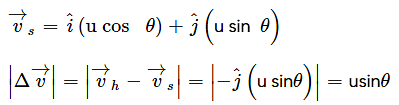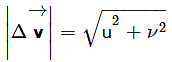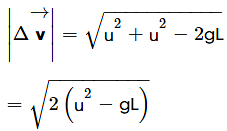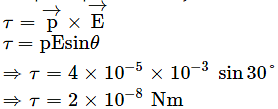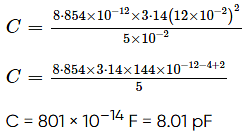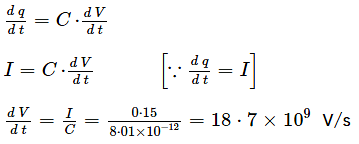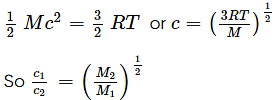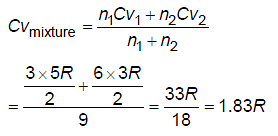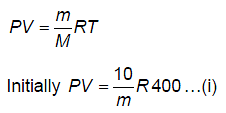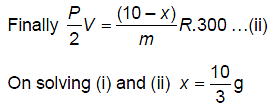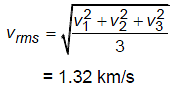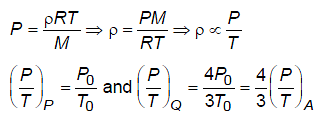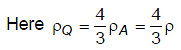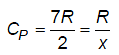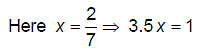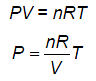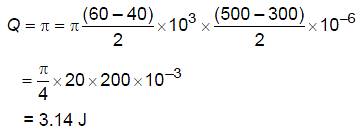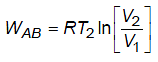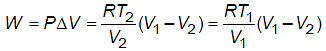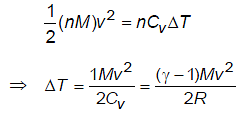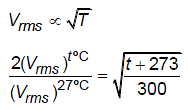NEET Practice Test - 1 - NEET MCQ
30 Questions MCQ Test NEET Mock Test Series 2025 - NEET Practice Test - 1
According to Newton, the viscous force acting between liquid layers of area A and velocity gradient Δv/Δz is given by 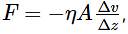 where η is constant called coefficient of viscosity. The dimensional formula of η is:
where η is constant called coefficient of viscosity. The dimensional formula of η is:
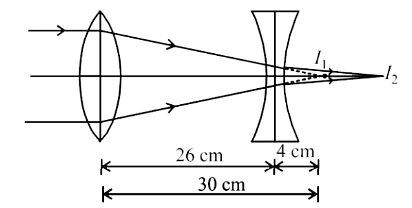
The size of the image of an object, which is at infinity, as formed by a convex lens of focal length 30 cm is 2 cm. If a concave lens of focal length 20 cm is placed between the convex lens and image at a distance of 26 cm from the convex lens, calculate the new size of the image.
The potential energy of a particle varies as
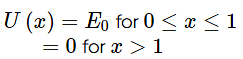
For 0≤ x ≤ 1, the de-Broglie wavelength is λ1 and for x > 1 the de-Broglie wavelength is λ2. The total energy of the particle is 2E0. Find λ1/λ2
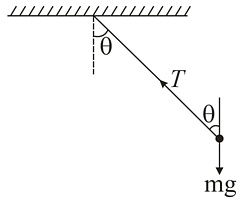
A car is moving in a circular horizontal track of radius 10 m with a constant speed of 10 m s−1. A plumb bob is suspended from the roof of the car by a light rigid rod. The angle made by the rod with the vertical is
(Take g=10 m s−2)
Two metallic wires of the same material have the same length but cross-sectional area is in the ratio 1:2. They are connected (i) in series and (ii) in parallel. Compare the drift velocities of electrons in the two wires in both the cases (i) and (ii).
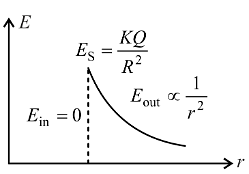
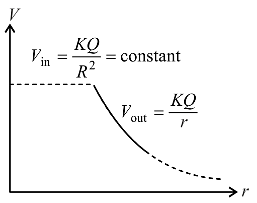
Consider a thin spherical shell of radius R with centre at the origin, carrying uniform positive surface charge density. The variation of the magnitude of the electric field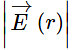 and the electric potential V(r) with the distance r from the centre, is best represented by which graph?
and the electric potential V(r) with the distance r from the centre, is best represented by which graph?
The time period of an earth satellite in circular orbit is independent of
A particle is fired with velocity u making angle θ with the horizontal. What is the magnitude of change in velocity when it is at the highest point?
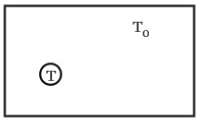
A black body of temperature T is inside a chamber of temperature T0. Now the closed chamber is slightly opened to the sun such that the temperature of black body (T) and chamber (T0) remains constant. Then,
A stone tied to a string of length L is whirled in a vertical circle with the other end of the string at the centre. At a certain instant of time, the stone is at its lowest position, and has a speed u. The magnitude of the change in its velocity as it reaches a position, where the string is horizontal, is
Following graph is showing the variation of potential energy between a pair of nucleons as a function of their separation. Indicate the regions A and B.
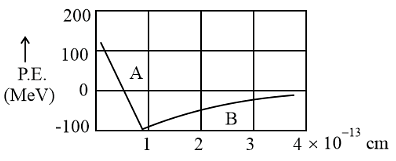
Two nucleons are at a separation of one Fermi. Protons have a charge of +1.6×10−19C. The net nuclear force between them is F1, if both are neutrons, F2, if both are protons F3, if one is proton and the other is neutron, Then :
An electric dipole of dipole moment 4×10−5 Cm is placed in a uniform electric field of 10−3 NC−1 making an angle of 30° with the direction of field. Determine the torque exerted by the electric field on the dipole.
Figure shows a capacitor made of two circular plates each of radius 12 cm and separated by 5.0 cm. The capacitor is being charged by an external source (not shown in the figure). The charging current is constant and equal to 0.15 A.
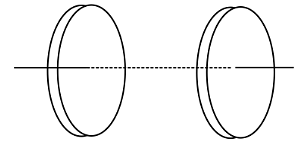
Calculate the capacitance and the rate of charge of potential difference between the plates.
If the molecular weight of two gases are M1 and M2, then at a temperature the ratio of RMS velocity c1 and c2 will be
The change in internal energy of a thermodynamical system which has absorbed 5000 cal of heat and does 5000 J of work is
Three moles of nitrogen is mixed with six moles of helium. The effective molar specific heat of the mixture at constant volume is
When the door of a refrigerator is kept open then the room temperature
A vessel has 10 g of hydrogen gas at pressure P and temperature 400 K. A small hole is made in it so that hydrogen leaks out. How much hydrogen leaks out if the final pressure becomes half and temperature becomes 3/4th of its previous value?
If three molecules of oxygen at 27ºC have velocities 0.5 km/s, 1 km/s and 2 km/s respectively. Then their root mean square speed will be
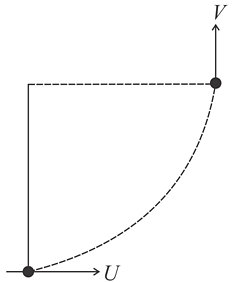
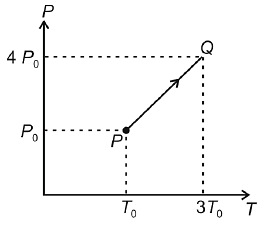
Temperature versus pressure graph of an ideal gas is shown in the figure. If density of gas at point P is then density of gas at point Q will be
For a rigid diatomic molecules, molar specific heat at constant pressure is given by CP = R/x, where R is universal gas constant and x is number value. Then 3.5x will be
Which of the following is not an assumption of kinetic theory of gases?
Which of the following law of thermodynamics forms the basis for the definition of temperature?
Which of the following diagram represent the isometric process correctly?
The work done by the system in the cyclic process as shown in figure is
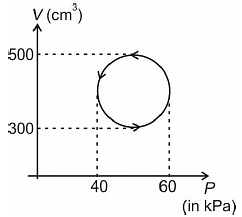
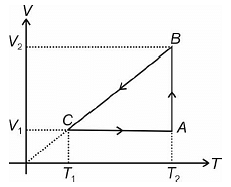
A cyclic process for one mole of an ideal gas is shown in the V–T diagram. The work done by the gas in AB, BC and CA respectively are
A box of negligible mass containing n moles of an ideal gas of molar mass M and adiabatic exponent moves with constant speed v on a smooth horizontal surface. If box suddenly stops, then change in temperature of gas will be
If CP and CV denoted the specific heat of unit gram mass of nitrogen at constant pressure and volume respectively, then (R is universal gas constant)
At what temperature the root mean square speed of molecules of a gas is twice of its root mean square speed at 27ºC?
|
1 videos|26 docs|111 tests
|