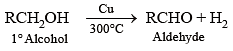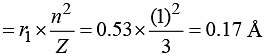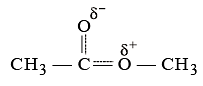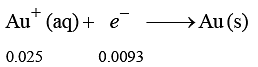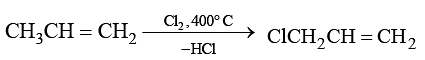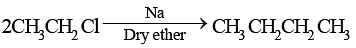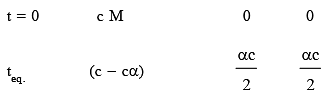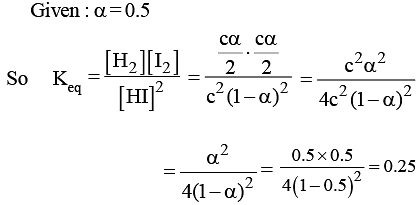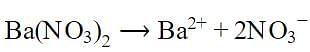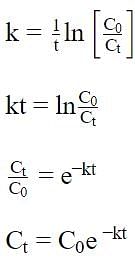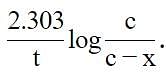NEET Practice Test - 11 - NEET MCQ
30 Questions MCQ Test NEET Mock Test Series 2025 - NEET Practice Test - 11
What is formed when a primary alcohol undergoes catalytic dehydrogenation?
The radius of the hydrogen atom in the ground state is 0.53 Å. The radius of Li2+ ion (atomic number = 3) in a similar state is
Match the vitamin of column I with deficiency disease given in column II

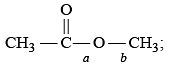
An ether is more volatile than an alcohol having the same molecular formula. This is due to
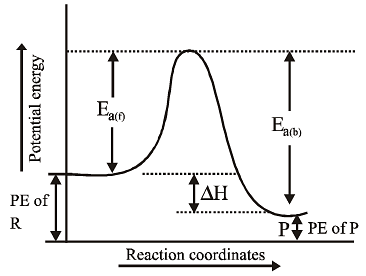
An exothermic reaction A → B has an activation energy of 17 kJ per mole of A. The heat of the reaction is 40 kJ. Calculate the activation energy for the reverse reaction B → A.
To which of the following is Bohr's theory applicable:
(I) He+
(II) Li2+
(III) Tritium
(IV) Be2+
The correct combination is:
The transition metal ions responsible for color in ruby and emerald are, respectively:
250 mL of a waste solution obtained from the workshop of a goldsmith contains 0.1 M AgNO3 and 0.1 M AuCl. The solution was electrolyzed at 2 V by passing a current of 1 A for 15 minutes.
The metal/metals electrodeposited will be :
![]()
Molecule which contains 4 bonded pairs and 2 lone pairs of electrons on the central atom is:
When chlorine is passed through propene at 400°C, which of the following is formed?
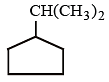
Which of the following compounds can best be prepared by Wurtz reaction?
At a certain temperature, only 50% HI is dissociated at equilibrium in the following reaction: 2HI(g) ⇌ H2(g) + I2(g). The equilibrium constant for this reaction is:
The vapour pressure of water at 300 K in a closed container is 0.4 atm. If the volume of the container is doubled, its vapour pressure at 300 K will be:
The number of hydrogen atoms present in 25.6 g of sucrose (C12H22O11) which has a molar mass of 342.3 g is:
An aqueous solution of the following concentration of Acetic acid is the best conductor.
Number of ions present in 1 ml of 0.1 M barium nitrate solution is:
In the lowest energy level of hydrogen atom, electron has an angular momentum equal to:
The size of particles in suspension, true solution and colloidal solution varies in the order
A system absorbs 10 kJ of heat and does 4 kJ of work. The internal energy of the system
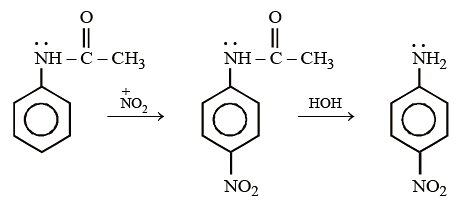
Acetanilide on nitration followed by alkaline hydrolysis mainly gives –
Which one of the following octahedral complexes will not show geometric isomerism? (A and B are monodentate ligands)
Which of the following ions has the maximum magnetic moment?
Aluminum oxide may be electrolysed at 1000 °C to furnish aluminum metal (At. Mass = 27 amu; 1 Faraday = 96,500 Coulombs). The cathode reaction is Al3+ + 3e– → Al
To prepare 5.12kg of aluminum metal by this method would require
If Co = initial concentration of the reactant, Ct = concentration of the reactant at time t and k = rate constant of the reaction, then the equation applicable for a first order reaction is:
|
1 videos|26 docs|111 tests
|