NEET Practice Test - 23 - NEET MCQ
30 Questions MCQ Test - NEET Practice Test - 23
The standard enthalpy of formation (∆fH°298) for methane, CH4 is – 74.9 kJ mol–1. In order to calculate the average energy given out in the formation of a C – H bond from this it is necessary to know which one of the following?
Addition of HI to double bond of propene yields isopropyl iodide and not n-propyl iodide as the major product, because addition proceeds through
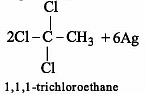
The major organic compound formed by the reaction of 1, 1, 1-trichloroethane with silver powder is:
The activation energy for a hypothetical reaction, A → Product, is 12.49 kcal/mole. If temperature is raised from 295 to 305, the rate of reaction increased by
Which one of the following pairs of molecules will have permanent dipole moments for both members?
An unknown metal M displaces nickel from nickel (II) sulphate solution but does not displace manganese from manganese sulphate solution. Which order represents the correct order of reducing power?
At STP, the order of root mean square speed of molecules H2, N2, O2 and HBr is
Match list I with list II and select the correct answer using the codes given below the lists:

The radius of the second Bohr orbit for hydrogen atoms is : (Plank's const. h = 6.6262 × 10–34 Js ; mass of electron = 9.1091 × 10–31 kg ; charge of electron e = 1.60210 × 10–19 C ; permittivity of vacuum ε0 = 8.854185 × 10–12 kg–1 m–3 A2)
The value of the ‘spin only’ magnetic moment for one of the following configurations is 2.84 BM.
The correct one is
CsCl crystallises in a body centered cubic lattice.
If ‘a’ is its edge length then which of the following expressions is correct?
The ether that undergoes electrophilic substitution reactions is
Number of electrons transferred in each case when KMnO4 acts as an oxidising agent to give MnO2, Mn2+, Mn(OH)3 and MnO42- are respectively
Consider the following changes
![]()
The energy required to pull out the two electrons are E1 and E2 respectively. The correct relationship between two energies would be
Which of the following is not formed when glycerol reacts with HI?
For preparing a buffer solution of pH 6 by mixing sodium acetate and acetic acid, the ratio of the concentration of salt and acid should be (Ka = 10–5)
BCl3 is a planar molecule whereas NCl3 is pyramidal because
An organic compound, C3H6O does not give a precipitate with 2,4-dinitrophenyl-hydrazine reagent and does not react with metallic sodium.
It could be
An element has a bcc structure having unit cells 12.08 × 1023. The number of atoms in these cells is
From the following statements regarding H2O2, choose the incorrect statement: