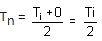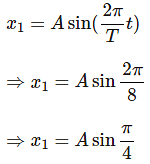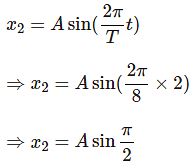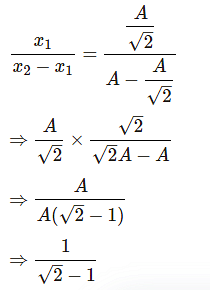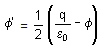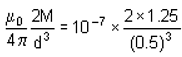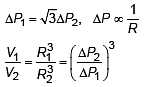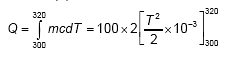NEET Practice Test - 4 - NEET MCQ
30 Questions MCQ Test NEET Mock Test Series 2025 - NEET Practice Test - 4
What will be the value of force on an electron moving with velocity 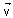 = 2.5 106
= 2.5 106 m/s in a magnetic field
m/s in a magnetic field 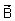 =
= 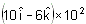 Wb/m2, if charge on an electron e is 1.6 x 10-19 C?
Wb/m2, if charge on an electron e is 1.6 x 10-19 C?
In a compound microscope, the objective and the eyepiece have focal lengths of 1 cm and 5 cm, respectively, and both are kept at a distance of 20 cm. If the final image is formed at the least distance of distinct vision from the eyepiece, find the total magnification.
Three thermally radiating carbon black coated discs A, B and C with radii 2 m, 4 m and 6 m have maximum intensity wavelengths 300 nm, 400 nm and 500 nm, respectively. If the powers radiated by them are Qa, Qb and Qc, respectively, then
A small block is shot into each of the four tracks as shown below. Each of the frictionless tracks rises to the same height. The speed with which the block enters the tracks is the same in all the cases. At the highest point of the track, normal reaction is the maximum in
A uniform rod of length 'l' and mass 'm' is free to rotate in a vertical plane about 'A'. The rod, initially in horizontal position, is released. The initial angular acceleration of the rod is (Moment of inertia of rod about A is ml2 / 3.
The extension in a string obeying Hooke's law is 'x'. The speed of sound in the stretched string is 'v'. If the extension in the string is increased to 1.5x, the speed of sound will be
For a satellite moving in an orbit around Earth, the ratio of kinetic energy to potential energy is
An elastic ball is dropped from a height h and it rebounds many times from the floor. If the coefficient of restitution is e, the time interval between the second and the third impacts, is
If the cold junction of a thermo-couple is kept at 0oC and the hot junction is kept at ToC, then the relation between neutral temperature (Tn) and temperature of inversion (Ti) is
A substance absorbs an amount of heat Q1 in going from one state to another and releases an amount of heat Q2 in coming back from the second state to the first state. How much work is done by the substance?
The equation of a progressive wave is given by Y = 0.2 cos![]() . The distance is expressed in cm and the time in seconds. What will be the minimum distance between the two particles having the phase difference of π/2?
. The distance is expressed in cm and the time in seconds. What will be the minimum distance between the two particles having the phase difference of π/2?
The time period of a particle in simple harmonic motion is 8 seconds. At t = 0, it is at the mean position. The ratio of the distances travelled by it in the 1st second and the 2nd second is
A hollow cylinder has a charge q coulomb on its surface. If Φ is the electric flux in units of voltmeter associated with the curved surface B, the flux linked with the plane surface A in units of voltmeter will be
If 25 W, 220 V and 100 W, 220 V bulbs are connected in series across a 440 V line, then
The dipole moment of a short bar magnet is 1.25 ampere - metre2. The magnetic field on its axis at a distance of 0.5 metre from the centre of the magnet is
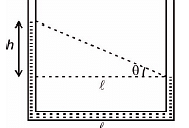
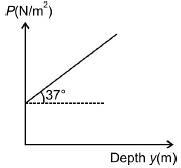
If the pressure due to liquid varies with the depth y as shown in figure, then the density of the liquid is (g = 10 m/s2)
A spherical black body with a radius of 12 cm radiates 450 watt power at 500 K. If the radius is halved and the temperature doubled, the power radiated in watt would be
A spherical body is thrown in a long column of viscous liquid with speed greater than terminal speed (vT). The correct variation of the speed (v) with time (t) is
The amount of liquid flowing per second in the tubes (1), (2) and (3) are 10 m3/s, 5 m3/s and 8 m3/s respectively. The velocity of liquid in the tube (4) having cross-section of 0.5 m2 is
Water rises to a height 20 cm in a capillary tube of cross-sectional area A. If the cross-sectional area of tube is 4A, then the height to which water will rise in a tube is
A U-tube containing a liquid is accelerated horizontally with a constant acceleration a. If the separation between the two vertical limbs is ![]() , then the difference in the heights of the liquid in the two arms is
, then the difference in the heights of the liquid in the two arms is
If a wire is stretched such that its length becomes double then
On the Fahrenheit scale, the temperature rise of nine degree is equivalent to how much degree rise in Celsius scale?
The excess of pressure inside the first soap bubble is √3 times that inside the second bubble. The ratio of volume of the first to that the second bubble is
100 g ice at 0°C is mixed with 10 g steam at 100°C, then mixture has
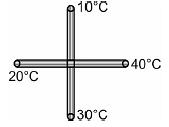
Four identical metal rods are connected as shown in figure. Assume there is no heat loss through side walls of the rods and no radiation loss, the junction temperature in steady state is
A metal wire of length L is suspended vertically from a rigid support. When a body of mass M is attached to the lower end of wire, the elongation of the wire is ![]() , then the incorrect statement is
, then the incorrect statement is
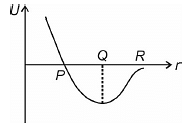
The variation in potential energy U between two molecules as a function of the distance between molecule has been shown in figure. The two molecules are
The specific heat of substance varies with temperature according to equation C = 2T × 10–3 cal/g K (T is absolute temperature). The amount of heat required to raise the temperature of 100 g of substance from 27°C to 47°C is
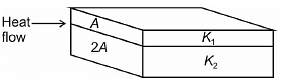
Two different metal slabs of identical length are joined in parallel. Their cross-sectional areas are A and 2A. If their thermal conductivity coefficients are K1 and K2 as shown then their equivalent conductivity for one dimensional steady heat transfer is
|
1 videos|26 docs|111 tests
|



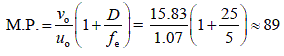

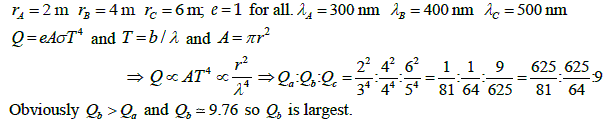
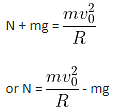
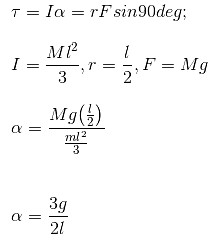



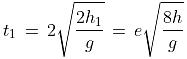
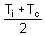 = neutral temperature
= neutral temperature