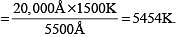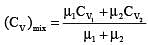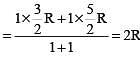Sample NEET Full Mock Test - NEET MCQ
30 Questions MCQ Test - Sample NEET Full Mock Test
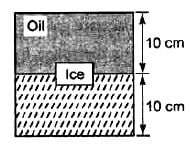
A cubical block of side 10 cm floats at the interface of an oil and water as shown in the figure. The density of oil is 0.6 g cm-3 and the lower face of ice cube is 2 cm below the interface. The pressure above that of the atmosphere at the lower face of the block is

Initial pressure and volume of a gas are P and V respectively. First it is expanded isothermally to volume 4V and then compressed adiabatically to volume V. The final pressure of gas will be (γ =1.5)
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
A gas is enclosed in a container which is then placed on a fast moving train. The temperature of the gas
In a thermodynamic process, the pressure of a fixed mass of a gas is changed in such a manner that the gas releases 20 J of heat and 8J of work is done on the gas. If the initial internal energy of the gas was 30 J, then the final internal energy will be
Five identical rods are joined as shown in figure. Point A and C are maintained at temperature 120°C and 20°C respectively. The temperature of junction B will be
Four massless springs whose force constants are 2k, 2k, k and 2k respectively are attached to a mass M kept on a frictionless plane (as shown in figure). If the mass M is displaced in the horizontal direction, then the frequency of oscillation of the system is
A wave travelling along positive x- axis is given by y = A sin(ωt − kx) . If it is reflected from rigid boundary at x = 0, such that 80% amplitude is reflected, then equation of reflected wave is
A point charge is surrounded symmetrically by six identical charges at distance r as shown in the figure. How much work is done by the forces of electrostatic repulsion when the point charge q at the centre is removed and placed at infinity
A conducting sphere of radius R, and carrying a charge q is joined to a conducting sphere of radius 2R, and carrying a charge – 2q. The charge flowing between them will be
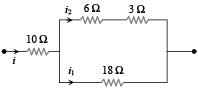
The figure here shows a portion of a circuit. What are the magnitude and direction of the current i in the lower right-hand wire
In the following circuit, 18Ω resistor develops a power of 2 J/sec due to current flowing through it. The power developed across 10Ω resistance is
A magnet is parallel to a uniform magnetic field. If it is rotated by 60°, the work done is 0.8 J. How much work is done in moving it 30° further
A straight wire of length L is bent into a semicircle. It is moved in a uniform magnetic field with speed v with diameter perpendicular to the field. The induced emf between the ends of the wire is
If A and B are identical bulbs which bulbs glows brighter (angular frequency of A.C. source = 100π)
A radioactive nucleus undergoes α - emission to form a stable element. What will be the recoil velocity of the daughter nucleus if V is the velocity of α - emission and A is the atomic mass of radioactive nucleus
In an electrical cable there is a single wire of radius 9 mm of Copper. Its resistance is 5 Ω . The cable is replaced by 6 different insulated Copper wires, the radius of each wire is 3 mm. Now the total resistance of the cable will be (l = 1)
An optical fibre communication system works on a wavelength of 1.3 μm. The number of subscribers it can feed if a channel requires 20 kHz are
A ray of light strikes a plane mirror M at an angle of 45° as shown in the figure. After reflection, the ray passes through a prism of refractive index 1.5 whose apex angle is 4°. The total angle through which the ray is deviated is
In Young’s double slit experiment, we get 60 fringes in the field of view of monochromatic light of wavelength 4000Å. If we use monochromatic light of wavelength 6000Å, then the number of fringes obtained in the same field of view is
Two discs of moment of inertia I1 and I2 and angular speeds ω1 and ω2 are rotating along collinear axes passing through their centre of mass and perpendicular to their plane. If the two are made to rotate together along the same axis the rotational KE of system will be
The bob of a simple pendulum is displaced from its equilibrium position O to a position Q which is at height h above O and the bob is then released. Assuming the mass of the bob to be m and time period of oscillations to be 2.0 sec, the tension in the string when the bob passes through O is
The acceleration due to gravity about the earth's surface would be half of its value on the surface of the earth at an altitude of (R = 4000 mile)
A thermodynamic system is taken through the cycle PQRSP. The net work done by the system is
A sound wave of wavelength 32 cm enters the tube at S as shown in the figure. Then the smallest radius r so that a minimum of sound is heard at detector D is
In an LCR circuit R = 100 ohm. When capacitance C is removed, the current lags behind the voltage by π / 3. When inductance L is removed, the current leads the voltage by π / 3. The impedance of the circuit is
If the magnetic dipole moment of an atom of diamagnetic material, paramagnetic material and ferromagnetic material is denoted by μd,μp,μf respectively then
Moment of inertia of a uniform circular disc about a diameter is I. Its moment of inertia about an axis perpendicular to its plane and passing through a point on its rim will be
A body at 1500 K emits maximum energy at a wavelength 20,000 Å . If the Sun emits maximum energy at wavelength 5500 Å , then the temperature of Sun is
One mole of a monoatomic ideal gas is mixed with one mole of a diatomic ideal gas. The molar specific heat of the mixture at constant volume is


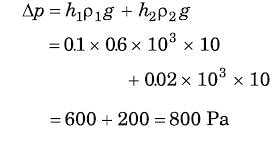
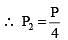
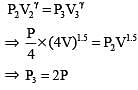
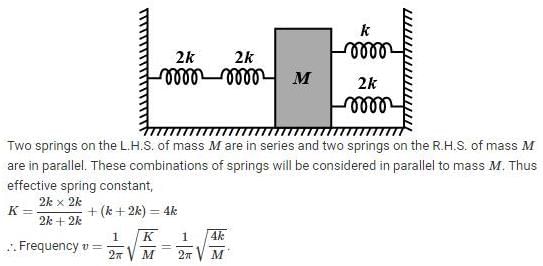
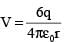
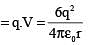
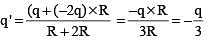
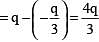
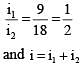
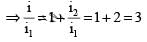
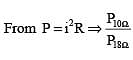
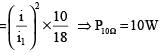
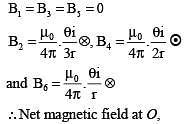
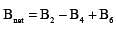
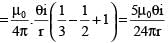
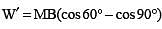
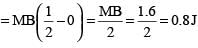
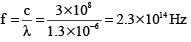
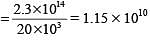
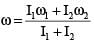
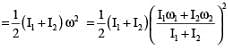
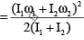
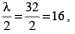

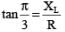 .....(i)
.....(i)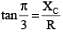 ....(ii)
....(ii)