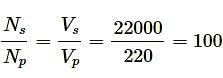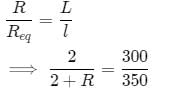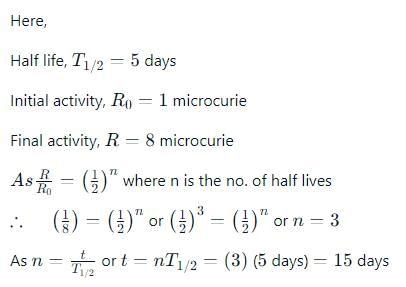Sample NEET Mock Test - NEET MCQ
30 Questions MCQ Test NEET Mock Test Series 2025 - Sample NEET Mock Test
A step up transformer connected to a 220 V AC line is to supply 22 kV for a neon sign in secondary circuit. In primary circuit a fuse wire is connected which is to blow when the current in the secondary circuit exceeds 10mA. The turn ratio of the transformer is
An electron and a proton have the same wave length. Which one of the following statements is correct about them?
Evidence for the wave nature of light cannot be obtained from
Emission of electrons in the photoelectric effect is possible, if
A heavy mass is attached to a thin wire and is whirled in a vertical circle. The wire is most likely to break
If there is an increase in length by 0.1% due to stretching, the percentage increase in its resistance willbe
A 2 Ω resistor is connected inseries with R Ω resistor. This combination is connected across a cell. When the potential difference across 2 Ω resistor is balanced on a potentiometer wire, null point is obtained at a length of 300 cm. When the same procedure is repeated for R Ω resistor, null point is obtained at a length of 350 cm . Value of R is
An electron remains undeflected when passing perpendicular to mutually perpendicular electric and magnetic fields. If the magnetic field is 8 Gauss and elecric field is 4000 V/m, the velocity of the electron is
In a discharge tube at 0.2 mm of Hg pressure, there is a formation of
The de-Broglie wavelength of an electron in the first Bohr orbit is
Which of the following electromagnetic waves have the smallest wavelength?
Which of the following radiations has the least wavelength?
An L.C. circuit contains 10 mH inductor and a 25 μ F capacitor. The resistance of the circuit is negligible. The energy stored in the circuit is completely magnetic at time (in milli seconds) being measured from the instant when the circuit is closed
At a point A, there is an electric field of 500 V-m⁻1 and potential difference of 3000 V. The distance between the point charge and the point A is
The escape velocity of an object on a planet whose radius is 4 times that of the earth and acceleration due to gravity 9 times that on the earth is
A gas exerts pressure on the walls of the container, because the gas molecules
Two capacitors of capacity 4 μF and 6 μF are connected in series and a battery is connected to the combination the energy stored is E1. If they are connected in parallel and if the same battery is connected to this combination the energy stored is E2. The ratio of E1 and E2 is
A body takes 5 minutes for cooling from 50°C to 40°C. Its temperature comes down to 33.33°C in next 5 minutes. Temperature of surroundings is
A stone of mass m is tied to a string and is moved in vertical circle of radius r making n revolutions per minute. The total tension in the string when the stone is at lowest point is
A rectangular loop carrying current i is placed in a uniform magnetic field B. The area enclosed by the loop is A. If there are n turns in the loop, the torque acting on the loop is given by
In a deflection magnetometer experiment in tan A position, a short-bar magnet placed at 18 cm from the centre of the compass needle produces a deflection of 30° . If another magnet of same length but 16 times pole strength as that of first magnet is placed in tan B position at 36cm, the deflection will be
The pole strength of a 12cm long bar magnet is 20 A-m. The magnetic induction at a point 10cm away from the centre of the magnet on its axial line is ( μ 0 4 π = 10-7 Hm-1 )
If iron pieces loaded in a ship, are thrown into the water, then level of water will
The Young's modulus of a wire of length (L) and radius (r) is Y N-m⁻2. If the length is reduced to L/2 and radius to r/2, then Young's modulus for the wire will be
The number of beta particles emitted by a radioactive substance is twice the number of alpha particles emitted by it. The resulting daughter is an:
The above is a plot of binding energy per nucleon Eb, against the nuclear mass M;, A, B, C, D, E, F correspond to different nuclei. Consider four reactions:
(i) A + B → C + ε
(ii) C → A + B + ε
(iii) D + E → F + ε and
(iv) F → D + E + ε
Where ε is the energy released ? In which reactions is ε positive?
A radio active sample has a half life of 5 days. To decay from 8 micro curies to 1 micro-curie, the number of days taken will be
If a simple pendulum oscillates with an amplitude 50 mm and time period of 2 s, then its maximum velocity, is
For what distance is ray optics a good approximation when the aperture is 4 mm wide and the wavelength is 500 nm?
|
1 videos|26 docs|111 tests
|