NEET Exam > NEET Tests > Test: Chemistry Mock Test - 5 - NEET MCQ
Test: Chemistry Mock Test - 5 - NEET MCQ
Test Description
30 Questions MCQ Test - Test: Chemistry Mock Test - 5
Test: Chemistry Mock Test - 5 for NEET 2025 is part of NEET preparation. The Test: Chemistry Mock Test - 5 questions and answers have been prepared
according to the NEET exam syllabus.The Test: Chemistry Mock Test - 5 MCQs are made for NEET 2025 Exam.
Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Test: Chemistry Mock Test - 5 below.
Solutions of Test: Chemistry Mock Test - 5 questions in English are available as part of our course for NEET & Test: Chemistry Mock Test - 5 solutions in
Hindi for NEET course.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free. Attempt Test: Chemistry Mock Test - 5 | 45 questions in 45 minutes | Mock test for NEET preparation | Free important questions MCQ to study for NEET Exam | Download free PDF with solutions
Test: Chemistry Mock Test - 5 - Question 1
Which of the following would react most readily with nucleophiles?
Detailed Solution for Test: Chemistry Mock Test - 5 - Question 1
Detailed Solution for Test: Chemistry Mock Test - 5 - Question 2
Detailed Solution for Test: Chemistry Mock Test - 5 - Question 3
Test: Chemistry Mock Test - 5 - Question 4
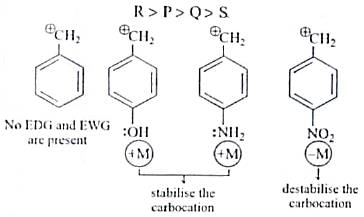
The decreasing order of stability of following cation is
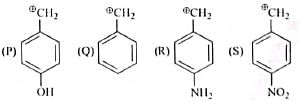
Detailed Solution for Test: Chemistry Mock Test - 5 - Question 4
Test: Chemistry Mock Test - 5 - Question 5
The ratio of the molar amounts of H₂S needed to precipitate the metal ions from 20 mL each of 1M Cd(NO₃)₂ and 0.5 M CuSO₄ is
Detailed Solution for Test: Chemistry Mock Test - 5 - Question 5
Test: Chemistry Mock Test - 5 - Question 6
On heating with conc. HNO₃, proteins give yellow colour. This test is called
Test: Chemistry Mock Test - 5 - Question 8
The work done during expansion of a gas from a volume of 4 dm3 to 6 dm3 against a constant external pressure of 3 atm (1 L-atm = 101.32 J)
Test: Chemistry Mock Test - 5 - Question 9
In which of the following case,does the reaction go farthest completion ?
Detailed Solution for Test: Chemistry Mock Test - 5 - Question 9
Test: Chemistry Mock Test - 5 - Question 11
Variation of heat of reaction with temperature is known as
Test: Chemistry Mock Test - 5 - Question 14
The atomic radius of elements of which of these would be nearly same
Detailed Solution for Test: Chemistry Mock Test - 5 - Question 14
Test: Chemistry Mock Test - 5 - Question 16
Which of the following transition metal has the highest melting point?
Test: Chemistry Mock Test - 5 - Question 17
The element, which forms oxides in all oxidation states + 1 to + 5, is
Test: Chemistry Mock Test - 5 - Question 19
If 0.44 g of a colourless oxide of nitrogen occupies 224 ml at 1520 mm hg and 273oC, then the compound is
Test: Chemistry Mock Test - 5 - Question 20
Which one of the following metallic hydroxides does not dissolve in sodium hydroxide solution?
Test: Chemistry Mock Test - 5 - Question 23
With respect to protonic acids, which of the following statements is correct?
Test: Chemistry Mock Test - 5 - Question 24
Aqueous solution of hydrogen sulphide and sulphur dioxide when mixed together ,yield
Test: Chemistry Mock Test - 5 - Question 25
Iodine readily dissolves in potassium iodide solution giving
Test: Chemistry Mock Test - 5 - Question 27
The test used for identifying carbon-carbon double bond in an alkene
Test: Chemistry Mock Test - 5 - Question 28
Which one of the following processes will produce hard water ?
View more questions
Information about Test: Chemistry Mock Test - 5 Page
In this test you can find the Exam questions for Test: Chemistry Mock Test - 5 solved & explained in the simplest way possible.
Besides giving Questions and answers for Test: Chemistry Mock Test - 5, EduRev gives you an ample number of Online tests for practice
Download as PDF


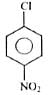 reacts (rapidly) with nucleophiles.
reacts (rapidly) with nucleophiles.