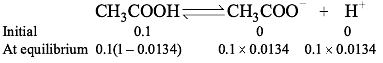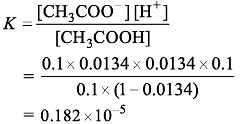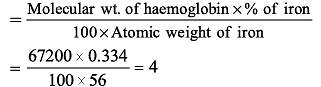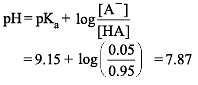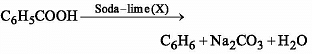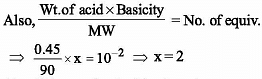NEET Practice Test - 20 - NEET MCQ
30 Questions MCQ Test NEET Mock Test Series 2025 - NEET Practice Test - 20
Which of the following statements is incorrect regarding dehydrohalogenation of alkenes ?
Which one of the following complexes will consume more equivalents of aqueous solution of AgNO3?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
If 0.1 M of a weak acid is taken, and its percentage of degree of ionization is 1.34%, then its ionization constant will be :
Which of the following statement(s) is/are correct?
(i) The atomic and ionic radii of alkaline earth metals are smaller than those of the corresponding alkali metals in the same periods.
(ii) Second ionisation enthalpies of the alkaline earth metals are smaller than those of the corresponding alkali metals.
(iii) Compounds of alkaline earth metals are more extensively hydrated than those of alkali metals
Haemoglobin contains 0.334% of iron by weight.
The molecular weight of haemoglobin is approximately 67200. The number of iron atoms (at. wt. of Fe is 56) present in one molecule of haemoglobin are
The reagent (s) which can be used to distinguish acetophenone from benzophenone is (are):
Among the following complexes, optical activity is possible in
The pKa of an amino acid is 9.15. At what pH amino acid is 5% dissociated ?
T50 of first -order reaction is 10 min. Starting with 10 mol L–1, rate after 20 min is
SnO2 is taken in basic medium and current is passed. Colloidal sol migrates towards
The basicity of aniline is less than that of cyclohexylamine. This is due to
Benzoic acid gives benzene on being heated with X and phenol gives benzene on being heated with Y. Therefore X and Y are respectively
Formation of a solution from two components can be considered as
(i) pure solvent → separated solvent molecules, ΔH1
(ii) pure solute → separated solute molecules, ΔH2
(iii) separated solvent and solute molecules → solution, ΔH3
Solution so formed will be ideal if
Which of the following pairs of compounds are positional isomers ?
In which of the following regions hydrogen and helium are found :
The cation that will not be precipitated by H2S in the presence of dil. HCl is :
AB crystallizes in a body centered cubic lattice with edge length ‘a’ equal to 387 pm. The distance between two oppositely charged ions in the lattice is :
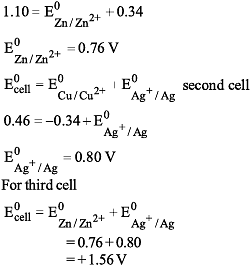
For the cell
Zn | Zn2+ (1M) || Cu2+ (1M) | Cu, E°cell is 1.10 V, E°Cu2+/Cu = 0.34 V and for the cell Cu | Cu2+ (1M) | | Ag+ (1M) | Ag, E°cell = 0.46 V hence, E°cell of the cell Zn | Zn2+ (1M) || Ag+ (1M) | Ag is
The correct order of ionic radii of Y3+, La3+, Eu3+ and Lu3+ is
In the froth floatation process for the purification of ores, the ore particles float because :
Which of the following organometallic compound is σ and π bonded?
The correct order of the decreasing ionic radii among the following isoelectronic species are :
0.45 g of acid of molecular weight 90 was neutralized by 20 ml. of a 0.5N caustic potash.
The basicity of an acid is
|
1 videos|26 docs|121 tests
|
|
1 videos|26 docs|121 tests
|