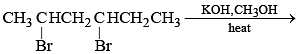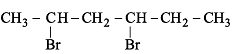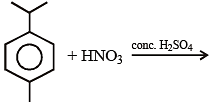NEET Practice Test - 22 - NEET MCQ
30 Questions MCQ Test NEET Mock Test Series 2025 - NEET Practice Test - 22
If the principal quantum number n = 6, the correct sequence of filling of electrons will be :
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The four quantum numbers that could identify the third 3p electron in sulphur are
Explanation
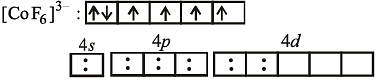
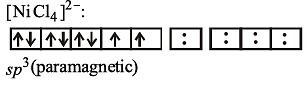
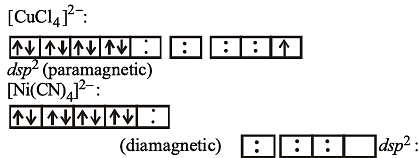
Paramagnetism of Cr (Z = 24), Mn2+ (Z = 25) and Fe3+ (Z = 26) are x, y and z respectively. They are in the order
Fluorine does not show highest oxidation state opposite to other halogens, because
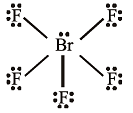
Shapes of certain interhalogen compounds are stated below. Which one of them is not correctly stated?
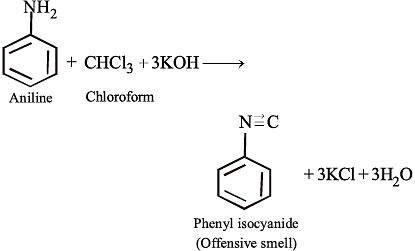
Aniline, chloroform and alcoholic KOH react to produce a bad smelling substance which is
The chemical reaction, 2AgCl(s) + H2 (g) → 2HCl(aq)+ 2Ag(s) taking place in a galvanic cell is represented by the notation
Complexes [Co(NH3)5 SO4] Br and 4 [Co(NH3)5Br]SO4 can be distinguished by
When conc. HNO3 acts on our skin, the skin becomes yellow, because :
When NaCl is dopped with 1.0 × 10–3 mole of SrCl2, the number of cation vacancy is
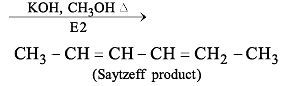
Which of the following represents a correct sequence of reducing power of the following elements?
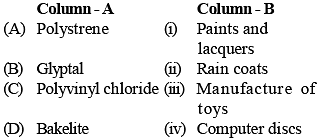
Match the polymers in column - A with their main uses in column - B and choose the correct answer:
The favourable condition for a process to be spontaneous is :
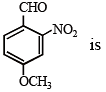
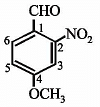
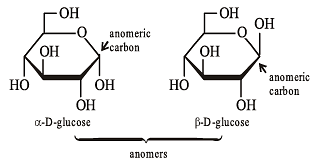
Which of the following compound is expected to be optically active?
Which of the following can be predicted from electronegativity values of elements ?
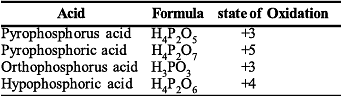
The pair in which phosphorous atoms have a formal oxidation state of + 3 is :
In CsCl, if coordination number of Cs+ is 8, then coordination number of Cl– ion is :
|
1 videos|26 docs|121 tests
|
|
1 videos|26 docs|121 tests
|