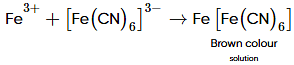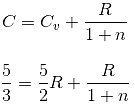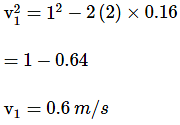JEE Advanced Practice Test- 1 - JEE MCQ
30 Questions MCQ Test Mock Tests for JEE Main and Advanced 2025 - JEE Advanced Practice Test- 1
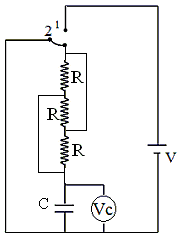
The switch in circuit shifts from 1 to 2 when VC > 2V/3 and goes back to 1 from 2 when VC < v/3.="" the="" voltmetre="" reads="" voltage="" as="" plotted.="" what="" is="" the="" period="" t="" of="" the="" wave="" form="" in="" terms="" of="" r="" and="" />



For a thermodynamic system, the pressure, volume and temperature are related as P =  , where is a positive constant. The work done for this system in a constant pressure process, if the temperature changes from To to 2To, is
, where is a positive constant. The work done for this system in a constant pressure process, if the temperature changes from To to 2To, is
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
One mole of a perfect gas expands isothermally to ten times its original volume.The change in entropy is :
A small block of mass of 0.1 kg lies on a fixed inclined plane PQ which makes an angle θ with the horizontal. A horizontal force of 1 N acts on the block through its centre of mass as shown in the figure. The block may remains stationary if (take g = 10 m s−2)

There are four sphere of each with radius as a, b, c, d, each sphere is solid, insulating and uniformly charged. Variation of electric field with distance rr from the centre is given. Straight portion of curve c and d is overlapping and straight portion of curve a and b is overlapping.

In the figure, a man of true mass M is standing on a weighing machine placed in a cabin. The cabin is joined by a string with a body of mass m. Assuming no friction, and negligible mass of cabin and weighing machine. Then

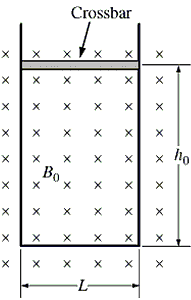
In the figure below, a closed loop is made of a U-shaped metallic wire of negligible resistance and a moving cross-bar of resistance R. The length of the cross-bar is L and has a mass m. It is initially placed at a distance h0 from the other end of the loop. The whole loop is placed in a uniform magnetic field of intensity Bo, as shown in the figure. Then,
Function x = A sin2 ωt + B cos2ωt + C sinωt cosωt represents SHM
In the circuit below, initially switch S1 is kept closed for a longer time and then switch S2 is closed. Which of the following statements is/are true?

Directions: The question has four choices, out of which ONE or MORE may be correct.
A composite block is made of slabs A, B, C, D and E of different thermal conductivities (given in terms of a constant K) and sizes (given in terms of length, L) as shown in the figure. All slabs are of same width. Heat 'Q' flows only from left to right through the blocks. Then in steady state,

An ideal gas (2 moles, diatomic) undergoes a thermodynamic process P.V-n = constant. For a change of temperature of 20 K, heat required is 200/3R (R is universal gas constant). The value of 'n' is
If wavelength associated with an electron revolving in nth orbit of a singly ionised He-atom is 14.976 Å, then the value of 'n' is
A bullet loses 1/x4 of its velocity in passing through a given plank of energy dissipating medium. If after passing through 8 such planks, the bullet comes to rest, the value of 'x' is
A string having length 0.5 m fixed at one end and other end is connected to a block of mass m = 2 kg as shown in figure. The string is set into vibrations which is represented by y = 4sin(πx/5)cos(50πt), where x and y are in cm and t is in second. The number of anti-nodes between point A and the fixed pulley is 2n, find n.

Two particles A and B of masses mm and 2m are connected through a rod of length l. The system lies on a smooth horizontal surface. An impulse of magnitude mv to the particle B is imparted through impact horizontally but perpendicular to the rod. The angular velocity of the rod just after impact is v / nl. Find 2n.

The friction coefficient between the horizontal surface and each of the blocks shown in the figure is 0.2. The collision between the blocks is perfectly elastic. Find the separation (in cm) between them when they come to rest.
Take g=10 m s-2.

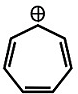
Which of the following is the correct order of stability of the given compounds?
When chlorine water is added to an aqueous solution of sodium halide in the presence of chloroform, a violet colouration is obtained. When more of chlorine water is added, the violet colour disappears and solution becomes colourless. This confirms that sodium halide is :
During the dehydration of alcohols, the hydrogen is removed from the carbon atom having a lesser number of hydrogen atoms to yield the most stable alkene as a major product. Identify the most stable alkene formed during the following conversion:

Directions: The question is Based on the following paragraph. This question has FOUR options, out of which ONLY ONE is correct.
A tertiary alcohol H upon acid catalysed dehydration gives a product I. Ozonolysis of I leads to compounds J and K. Compound J upon reaction with KOH gives benzyl alcohol and a compound L, whereas K on reaction with KOH gives only M.

Compound H is formed by the reaction of
Which of the following substances exhibit tautomerism
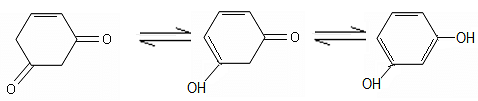
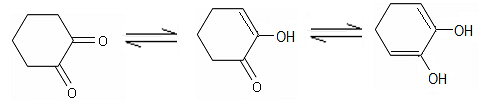
Observe the following sequence of reactions and select the option(s) that is/are true in this context.

A crystalline substance composed of Ba, Ti and O crystallises in a perovskite structure. This structure may be described as a cubic lattice, with barium ion occupying corners of the unit cell and oxide ions occupying the face centres of the unit cell. Which of the following statements is/are true?
Directions: The question has four choices, out of which ONE or MORE may be correct.
Extraction of metal from the ore cassiterite involves
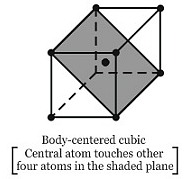
Amongst the following, the correct statements(s) is/are
Given is the arrangement of atoms in crystallographic plane.

Which plane correctly represent(s) the adjacent drawn structure ?
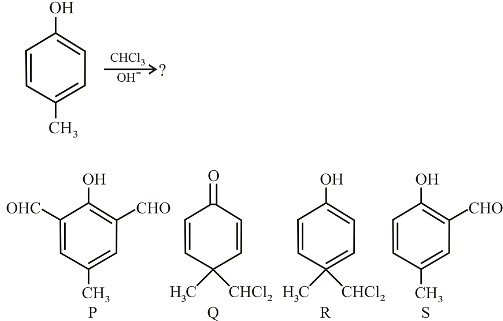
In the following reaction, the product(s) formed is(are):
|
357 docs|148 tests
|
|
357 docs|148 tests
|