JEE Advanced Practice Test- 4 - JEE MCQ
30 Questions MCQ Test Mock Tests for JEE Main and Advanced 2025 - JEE Advanced Practice Test- 4
A small ball thrown with an initial velocity μ directed at an angle θ = 37° above the horizontal
collides inelastically (e = 1/4) with a vertical massive wall moving with a uniform horizontal
velocity u/5 towards ball. After collision with the wall, the ball returns to the point from where it
was thrown. Neglect friction between ball and wall. The time t from beginning of motion of the ball
till the moment of its impact with the wall is (tan37° = 3/4)
collides inelastically (e = 1/4) with a vertical massive wall moving with a uniform horizontal
velocity u/5 towards ball. After collision with the wall, the ball returns to the point from where it
was thrown. Neglect friction between ball and wall. The time t from beginning of motion of the ball
till the moment of its impact with the wall is (tan37° = 3/4)
A cube of side l and mass M is placed on rough horizontal surface and the friction is sufficient so
that it will not move, if a constant force F = Mg is applied horizontally l/4 above the surface. Then
the torque due to normal force about center of the cube is equal to
that it will not move, if a constant force F = Mg is applied horizontally l/4 above the surface. Then
the torque due to normal force about center of the cube is equal to
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
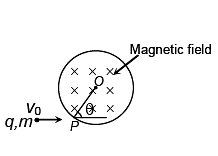
A particle of charge q and mass m is projected with a velocity v0 towards a circular region having uniform magnetic field B perpendicular and into the plane of paper from point P as shown in the figure. R is the radius and O is the centre of the circular region. If the line OP makes an angle θ with the direction of v0 then the value of v0 so that particle passes through O is
A ray of light from a liquid ( μ = √3 ) is incident on a system of two right angled prism of refractive indices √3 and √2 as shown. The ray suffers zero deviation when emerges into air from CD. The angle of incidence i is
This section contains 2 multiple choice questions. Each question has 4 choices (A), (B), (C) and (D) for its
answer, out of which ONE OR MORE is/are correct
Two blocks A and B each of mass 1/2 kg is connected by a massless inextensible string and kept on horizontal surface. Coefficient of friction between block and surface is shown in figure. A force F = kt (where k = 1 N/s and t is time in second) applied on A. Then (g = 10 m/s2)
A 4 μF capacitor is given 20 μC charge and is connected with an uncharged capacitor ofcapacitance 2 μF as shown in figure. When switch S is closed.
This section contains 2 paragraphs. Based upon one of paragraph 2 multiple choice questions and based
on the other paragraphs 3 multiple choice questions have to be answered. Each of these questions has
four choices (A), (B), (C) and (D) out of which ONLY ONE is correct
Paragraph for Question Nos. 10 and 11
A massive disc of radius R is mounted on a light axle with the axle held horizontal, the disc is made to
spin with angular speed ω0. It is then lowered gently on to a level table and released as soon as its rim
makes contact with the table top. The disc begins to move along the table, skidding and picking up speed
as it goes.
If μk is the coefficient of kinetic friction between the rim of the disc and table top, disc will continue
to skid for a time
A massive disc of radius R is mounted on a light axle with the axle held horizontal, the disc is made to
spin with angular speed ω0. It is then lowered gently on to a level table and released as soon as its rim
makes contact with the table top. The disc begins to move along the table, skidding and picking up speed
as it goes.
If initial kinetic energy of the disc is K, then the kinetic energy of the disc after it stars pure rolling
will be
Paragraph for Question Nos. 12 and 14
A uniform ring of mass m and radius R can rotate freely about an axis passing through centre C and perpendicular to plane of paper. Half of ring is positively charge and other half is negatively charge. Uniform electric field E0 is switched on along –ve x-axis (Axis are shown in figure) [magnitude of charge density λ]
The dipole moment of ring is
A uniform ring of mass m and radius R can rotate freely about an axis passing through centre C and perpendicular to plane of paper. Half of ring is positively charge and other half is negatively charge. Uniform electric field E0 is switched on along –ve x-axis (Axis are shown in figure) [magnitude of charge density λ]
The equilibrium of ring is
A uniform ring of mass m and radius R can rotate freely about an axis passing through centre C and perpendicular to plane of paper. Half of ring is positively charge and other half is negatively charge. Uniform electric field E0 is switched on along –ve x-axis (Axis are shown in figure) [magnitude of charge density λ]
If ring is slightly disturb from given position, find the angular speed of ring when it rotate by π/2.
This section contains 2 questions. Each question contains statements given in two columns, which have to be matched. The statements in Column I are labelled A, B, C and D, while the statements in Column II are labelled p, q, r, s and t. Any given statement in Column I can have correct matching with ONE OR MORE statement(s) in Column II. The appropriate bubbles corresponding to the answers to these questions have to be darkened as illustrated in the following example: If the correct matches are A – p, s and t; B – q and r; C – p and q; and D – s and t; then the correct darkening of bubbles will look like the following:
Three sound sources of same frequency f are arranged as shown in figure. S2 moves towards left
with speed v /n, S3 moves on a circular path of radius R with constant speed 2v/n
, and S1 performs S.H.M. with frequency 100v/nπ s-1and amplitude 1 cm. (n > 12 and v is speed of sound)
This section contains 2 questions. Each question contains statements given in two columns, which have to be matched. The statements in Column I are labelled A, B, C and D, while the statements in Column II are labelled p, q, r, s and t. Any given statement in Column I can have correct matching with ONE OR MORE statement(s) in Column II. The appropriate bubbles corresponding to the answers to these questions have to be darkened as illustrated in the following example: If the correct matches are A – p, s and t; B – q and r; C – p and q; and D – s and t; then the correct darkening of bubbles will look like the following:
This section contains 5 questions. Each question, when worked out will result in one integer from 0 to 9
(both inclusive).
A particle starts moving with velocity 10 m/s in a straight line under an acceleration varying linearly with time. Its velocity time graph is as shown in figure. Its velocity is maximum at t = 3 sec. Find the time (in sec) when the particle stops (tan 370 = ¾)
A block of mass 1 kg start moving at t = 0 with speed 2 m/s on rough horizontal surface with coefficient of friction 0.2. A horizontal force F is applied in the direction of velocity which varies with time shown in figure (B). Find the speed (in m/s) of particle at t = 3 s (g = 10 m/s2)
One end of a massless rope, which passes over a massless and frictionless pulley P is tied to a hook C while the other end is free. Maximum tension that the rope can bear is 360 N with what value of minimum safe acceleration (in ms–2) can a monkey of 60 kg move down on the rope
Two sources of emf 6V and internal resistance 3Ω and 2Ω are connected to an external resistance R as shown. If potential difference across battery A is zero, then find the value of R (in ohm)
This section contains 4 multiple choice questions numbered 1 to 4. Each question has 4 choices (A), (B),
(C) and (D), out of which ONLY ONE is correct
The formula of the following chain silicate (pyroxene) can be represented as
is a first order reaction. If A is strong mono protic acid and B is strong diprotic acid and reaction kinetic is studied by using a standard solution of NaOH required toneutralise reaction mixture at different instants of time. When initially we had taken sameconcentration of A in each case.Now it was observer that volume required after 971 sec was double that required initially, then thehalf life of reaction is:
Reduction potentials of some ions are given below. Arrange them in decreasing order of oxidizing power.
Ion ClO4− IO4− BrO4−
Reduction potential E∘/VE∘= 1.19VE∘= 1.65VE∘= 1.74V
Compound A and B, both were treated with NaOH , producing a single compound C:
This section contains 2 multiple choice questions. Each question has 4 choices (A), (B), (C) and (D) for its answer, out of which ONE OR MORE is/are correct
For the reaction NH3 . If Kp of the above reaction is 78.1 at 4000Cwhich is not correct:
20 mL of KOH solution was titrated with 0.20 mol L-1 H2SO4 solution in a conductively cell. The data obtained were plotted to give the graph shown below:
The concentration of the KOH solution was:
This section contains 2 paragraphs. Based upon one of paragraph 2 multiple choice questions and based on the other paragraphs 3 multiple choice questions have to be answered. Each of these questions has four choices (A), (B), (C) and (D) out of which ONLY ONE is correct
Paragraph for Question Nos. 10 and 11
The Ellingham diagram for the reduction of haematite and that of cuprous oxide given below;
On the basis of the above plot, answer the following two questions:
Choose the incorrect statement
The Ellingham diagram for the reduction of haematite and that of cuprous oxide given below;
On the basis of the above plot, answer the following two questions:
The incorrect statement amongst the following is
Paragraph for Question Nos. 12 and 14
Dolacaine, a local anesthetic, is a compound with molecular formula, C13 H20 O2 N2 . It is insoluble in water and dilute NaOH, but soluble in dil. HCl. Upon treatment with NaNO2 and HCl and then withβ -naphthol, a highly coloured solid is formed. When Novocaine is boiled with aqueous NaOH , it slowly dissolves. The alkaline solution is shaken with ether and layers are separated.
Acidification of the aqueous layer causes the precipitation of white solid ‘A’; continued addition of acid causes ‘A’ to redissolve. Upon isolation ‘A’ is found to have melting point of 185 -186° C and the formula C7 H7 O2 N : Evaporation of ether layer leaves a liquid ‘B’ of formula C6 H15 ON. with acetic anhydride gives 'C' C8 H17 O2 N which is insoluble in water and dilute bases, but soluble in dilute HCl. ‘B’ is found to be identical to the compound formed by the action of diethylamine on ethylene oxide.
When compound ‘B’ is treated with p – nitrobenzoyl chloride followed by moderate reduction with Ni/H2 ; the compound formed is
Dolacaine, a local anesthetic, is a compound with molecular formula, C13 H20 O2 N2 . It is insoluble in water and dilute NaOH, but soluble in dil. HCl. Upon treatment with NaNO2 and HCl and then withβ -naphthol, a highly coloured solid is formed. When Novocaine is boiled with aqueous NaOH , it slowly dissolves. The alkaline solution is shaken with ether and layers are separated.
Acidification of the aqueous layer causes the precipitation of white solid ‘A’; continued addition of acid causes ‘A’ to redissolve. Upon isolation ‘A’ is found to have melting point of 185 -186° C and the formula C7 H7 O2 N : Evaporation of ether layer leaves a liquid ‘B’ of formula C6 H15 ON. with acetic anhydride gives 'C' C8 H17 O2 N which is insoluble in water and dilute bases, but soluble in dilute HCl. ‘B’ is found to be identical to the compound formed by the action of diethylamine on ethylene oxide.
Dolacaine, a local anesthetic, is a compound with molecular formula, C13 H20 O2 N2 . It is insoluble in water and dilute NaOH, but soluble in dil. HCl. Upon treatment with NaNO2 and HCl and then withβ -naphthol, a highly coloured solid is formed. When Novocaine is boiled with aqueous NaOH , it slowly dissolves. The alkaline solution is shaken with ether and layers are separated.
Acidification of the aqueous layer causes the precipitation of white solid ‘A’; continued addition of acid causes ‘A’ to redissolve. Upon isolation ‘A’ is found to have melting point of 185 -186° C and the formula C7 H7 O2 N : Evaporation of ether layer leaves a liquid ‘B’ of formula C6 H15 ON. with acetic anhydride gives 'C' C8 H17 O2 N which is insoluble in water and dilute bases, but soluble in dilute HCl. ‘B’ is found to be identical to the compound formed by the action of diethylamine on ethylene oxide.
Compound B is having:
This section contains 2 questions. Each question contains statements given in two columns, which have to be matched. The statements in Column I are labelled A, B, C and D, while the statements in Column II are labelled p, q, r, s and t. Any given statement in Column I can have correct matching with ONE OR MORE statement(s) in Column II. The appropriate bubbles corresponding to the answers to these questions have to be darkened as illustrated in the following example:
If the correct matches are A – p, s and t; B – q and r; C – p and q; and D – s and t; then the correct darkening of bubbles will look like the following:
This section contains 2 questions. Each question contains statements given in two columns, which have to be matched. The statements in Column I are labelled A, B, C and D, while the statements in Column II are labelled p, q, r, s and t. Any given statement in Column I can have correct matching with ONE OR MORE statement(s) in Column II. The appropriate bubbles corresponding to the answers to these questions have to be darkened as illustrated in the following example:
If the correct matches are A – p, s and t; B – q and r; C – p and q; and D – s and t; then the correct darkening of bubbles will look like the following:
|
357 docs|148 tests
|
|
357 docs|148 tests
|

















