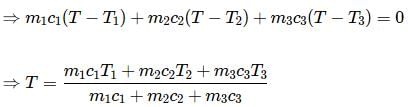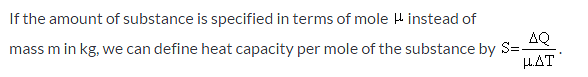Test: Calorimetry - NEET MCQ
10 Questions MCQ Test Physics Class 11 - Test: Calorimetry
Equal masses of three liquids of specific heats C1, C2 and C3 at temperatures t1, t2 and t3 respectively are mixed. If there is no change of state, the temperature of the mixture is
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which of the given relation is true for molar heat capacity of a substance?
A piece of iron of mass 100g is kept inside a furnace for a long time and then put in a calorimeter of water equivalent 10g containing 240g of water at 20°C. The mixture attains an equilibrium temperature of 60°C. Find the temperature of the furnace. Specific heat capacity of iron = 470J/kg-°C.
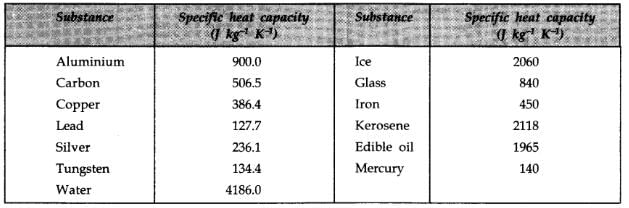
Among the following substances, which one has highest specific heat capacity?
The amount of heat required to raise the temperature of one mole of an ideal mono atomic gas through 2°C at constant pressure is (universal gas constant = R)
5 g of ice at 0° C is mixed with 10 g of water at 10° C. The temperature of the mixture is:
Which of the following relation is true for the specific heat capacity of substance?
According to law of calorimetry, which of the given relation is true?
|
97 videos|378 docs|103 tests
|