Test: SSC CGL Previous Year Questions: Chemistry (2023-20) - 3 - SSC CGL MCQ
30 Questions MCQ Test SSC CGL Previous Year Papers - Test: SSC CGL Previous Year Questions: Chemistry (2023-20) - 3
_________ is the heaviest naturally occurring element of the Periodic Table with an atomic weight of 238.
[SSC MTS 08/05/2023 (Evening)]
[SSC MTS 08/05/2023 (Evening)]
Which among the following is a noble chemical element of science periodic table?
[SSC MTS 19/06/2023 (Morning)]
[SSC MTS 19/06/2023 (Morning)]
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
In which group are the non-metal elements placed in a vertical column on the right side of the periodic table?
[SSC CGL 05/12/2022 (1st Shift)]
[SSC CGL 05/12/2022 (1st Shift)]
Which of the following types of elements is included in the leftmost group of the periodic table?
[SSC CPO 10/11/2022 (Evening)]
Which of the following elements has the largest atomic radii?
[SSC MTS 06/07/2022 (Morning)]
Identify the group of the periodic table to which cobalt belongs.
[SSC CHSL 25/05/2022 (Evening)]
Why does the effective nuclear charge experienced by valence electrons decrease down the group?
[SSC CHSL 10/06/2022 (Afternoon)]
What is the atomic mass of oxygen (expressed in ‘u’)?
[SSC CGL 18/04/2022 (Evening)]
Of the known elements in the periodic table, only ______ are gases under normal atmospheric conditions.
[SSC CGL 16/08/2021 (Afternoon)]
The elements of the groups 3 to 12 are called ______ elements or transition elements.
[SSC CGL 16/08/2021 (Afternoon)]
In the periodic table, the highly electronegative halogens and the highly electropositive alkali metals are separated by: [SSC CGL 16/08/2021 (Evening)]
Which of the following is used as a cooling medium for the Large Hadron Collider (LHC) and the superconducting magnets in MRI scanners and NMR spectrometers?
[SSC CGL 14/07/2023 (1st shift)]
One mole of an ideal gas occupies a volume of ______ litre at 273 K and 1 atm pressure.
[SSC CGL 19/04/2022 (Evening)]
Identify the odd term with respect to allotropes of carbon.
[SSC Stenographer 12/10/2023 (Evening)]
From the given options, choose the one which is NOT a product of the decomposition reaction of Lead nitrate.
[SSC CPO 05/10/2023 (Morning)]
Which of the following pairs of compounds - boiling point is correct?
I. Chloroform - 334K
II. Methane - 111K
[SSC CGL 19/07/2023 (4th shift)]
Which of the following acids makes up 55-80% of olive oil, making it a good choice for most cooking methods?
[SSC CGL 21/07/2023 (3rd shift)]
What is the product of a reaction of calcium carbonate, water and carbon dioxide?
[SSC CGL 08/12/2022 (1st Shift)]
Which of the following is correctly paired?
[SSC CGL 13/04/2022 (Evening)]
Which of the following is a property of Beryllium?
[SSC CGL 20/04/2022 (Evening)]
Which of the following is a polar molecule?
[SSC CHSL 09/08/2021 (Morning)]
Which of the following options are homogeneous mixtures?
[SSC CPO 04/10/2023 (Evening)]
What is the percentage composition of the element of H2SO3?
[SSC CGL 19/07/2023 (3rd shift)]
[SSC CHSL 09/03/2023 (1st Shift)]
[SSC CHSL 09/03/2023 (2nd Shift)]
Match List-I with List-II.
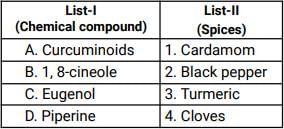
[SSC CGL Tier II 26/10/2023]
[SSC Stenographer 12/10/2023 (Afternoon)]
[SSC Stenographer 13/10/2023 (Afternoon)]
Which of the following is NOT a food preservative?
[SSC CPO 03/10/2023 (Evening)]
Which of the following is the product as a result of the process of hydrolysis of orthoclase?
[SSC CHSL 10/08/2023 (4th shift)]
|
316 docs|268 tests
|

















