Test: Common Ion Effect - NEET MCQ
10 Questions MCQ Test Topic-wise MCQ Tests for NEET - Test: Common Ion Effect
The following equilibrium exists in aqueous solution
CH3COOH 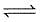 CH3COO- + H+
CH3COO- + H+
If dilute HCl is added:
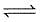 CH3COO- + H+
CH3COO- + H+If dilute HCl is added:
Consider KA, the potassium salt of a weak acid, HA. If a sample of KA is dissolved in water, with no other substances added, which of the following statements is true (approximately)?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
If the value of the solubility product for AgBr is 4.0 x 10-12 at 25°C, calculate the solubility of AgBr(s) in water.
Role of NH4Cl in qualitative analysis of third group cations
A mixture of NH4Cl and NH4OH shows no change in pH upon addition small amount of HCl. This is because it is
We eat a variety of foods still pH of our blood does not change every time. The reason is
For HF, pKa = 3.45. What is the pH of an aqueous buffer solution that is 0.1M HF (aq) and 0.300 M KF (aq)?
|
9 docs|1272 tests
|

















