31 Year NEET Previous Year Questions: Some Basic Principles & Techniques - 1 - NEET MCQ
28 Questions MCQ Test Chemistry Class 11 - 31 Year NEET Previous Year Questions: Some Basic Principles & Techniques - 1
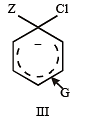
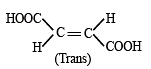
Which of the following compounds will exhibit cis-trans (geometrical) isomerism? [2009]
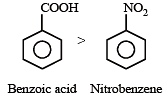
Given are cycloh exanol (I) a cetic acid (II), 2, 4, 6 – trinitrophenol (III) and phenol (IV). In these the order of decreasing acidic character will be :[2010]
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
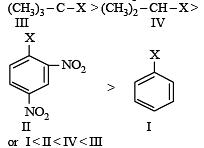
The correct order of increasing reactivity of C – X bond towards nucleophile in the following compounds is: [2010]
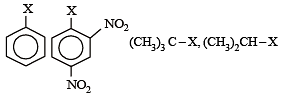
(I) (II) (III) (IV)
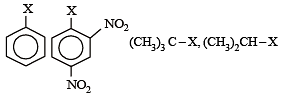
Among the given compounds , the most susceptible to nucleophilic attack at the carbonyl group is: [2010]
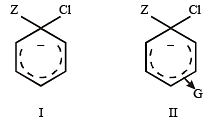
Which one is most reactive towards electrophilic reagent? [2010]
In the following the most stable conformation of n-butane is: [2010]
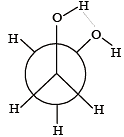
Which of the following conformers for ethylene glycol is most stable? [2010]
Which of the following species is not electrophilic in nature? [2010]
Which one of the following is most reactive towards electrophilic reagent ? [2011]
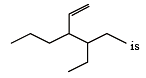
The correct IUPAC name of the compound
The correct order of increasing bond length of C – H, C – O, C – C and C = C is : [2011]
Which one is a nucleophilic substitution reaction among the following ? [2011]
Which of the following compounds undergoes nucleophilic substitution reaction most easily ?
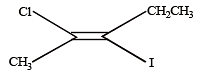
The IUPAC name of the following compound is
In Duma's method of estimation of nitrogen 0.35 g of an organic compound gave 55 mL of nitrogen collected at 300 K temperature and 715 mm pressure. The percentage composition of nitrogen in the compound would be : (Aqueous tension at 300 K = 15 mm) [2011]
The Lassaigne’s extract is boiled with conc.HNO3 while testing for halogens. By doing so it [2011]
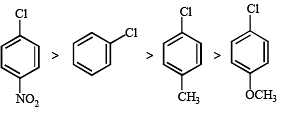
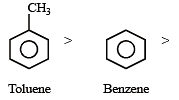
Among the following compounds the one that is most reactive towards electrophilic nitration is: [2012]
Which nomenclature is not according to IUPAC system? [2012]
Which of the following acids does not exhibit optical isomerism ? [2012]
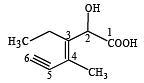
Structure of the compound whose IUPAC name is 3-ethyl-2-hydroxy-4-methylhex-3-en-5-ynoic acid is : [NEET 2013]
Some meta-directing substituents in aromatic substitution are given. Which one is most deactivating? [NEET 2013]
Which of the following compounds will not undergo Friedal-Craft’s reaction easily : [NEET 2013]
The structure of isobutyl group in an organic compound is : [NEET 2013]
Arr ange the following in increasing order of stability [NEET Kar. 2013]
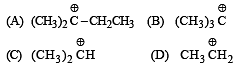
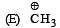
Given
I and II are [NEET Kar. 2013]
Homolytic fission of the following alkanes forms free radicals CH3 – CH3, CH3 – CH2 – CH3, (CH3)2 CH – CH3, CH3 – CH2 – CH (CH3)2. Increasing order of stability of the radicals is [NEET Kar. 2013]
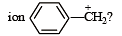
What is the hybridisation state of benzyl carbonium [NEET Kar. 2013]
Nitrogen detection in an organic compound is carried out by Lassaigne’s test. The blue colour formed corresponds to which of the following formulae? [NEET Kar. 2013]
|
129 videos|238 docs|88 tests
|


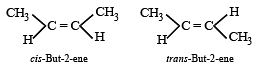
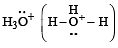 has a lone pair of electrons on oxygen atom, thus not an electrophile. Also octet is complete.
has a lone pair of electrons on oxygen atom, thus not an electrophile. Also octet is complete.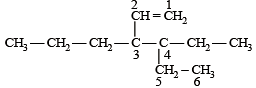
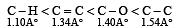
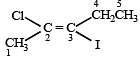
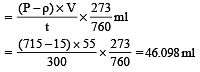
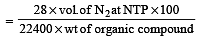
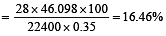
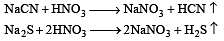
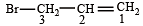 is 3-Bromoprop-1-ene.
is 3-Bromoprop-1-ene.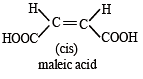
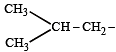 (iso-butyl group)
(iso-butyl group)














