Test: Chemical Kinetics - 3 - Chemistry MCQ
30 Questions MCQ Test Physical Chemistry - Test: Chemical Kinetics - 3
The energy of activation of a forward reaction is 50Kcal. The energy of activation of a backward reaction is:
The rate constant is numerically the same for three reactions of first, second and third orders respectively. When concentration is less than unity, the rate of reactions are, in order:
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The number of molecules of the reactants involved in a single stage of the reaction indicates
Rate of a reaction. A + B → Product is given below as a function of different initial concentrations of A and B.
The rate of the reaction is:
In a reaction: 2N2O5 → 4NO2 + O2 , the rate is expressed as
The relation among K1, K2 and K3 is:
Calculate order of reaction A → product , from the following data:
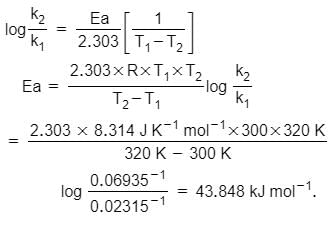
A first order reaction is 50% complete in 30mins at 270C and in 10mins at 470C. At 270C, the activation energy of the reaction in KJ/mole is:
What is the rate of simple reaction 2NO + O2 → 2 NO2 ; when the volume of the reaction vessel is doubled?
Which among the following procedures will lead to a change in rate constant ‘k’ of a reaction?
(I) A change in pressure
(II) Change in temperature
(III) Change in volume of the reaction vessel
(IV) An introduction of a catalyst
For a given set of reactions:
The rate constant for the decay of N2O5 depends on:
The gas phase decomposition of NOBr is second order in [NOBr], with k = 0.810 M–1 s–1 at 100C. We start with in a flask at 100C. How many seconds does it take to use up
2NOBr(g) → 2NO(g) + Br2(g)
Rate = k[NOBr]2
The concentration of a reactant undergoing decomposition was 0.1, 0.08 and 0.067 mol L–1 after 1.0, 2.0 and 3.0 hr respectively. The order of the reaction is:
Plots showing the variation of the rate constant (k) with temperature (T) are given below. The plot that follows Arrhenius equation is:
A reaction of first-order completed 90% in 90 minutes, hence, it is completed 50% in approximately.
At 350K, the effective rate constant for a gaseous reaction A → P , which has a Lindeman-Hinshelwood mechanism are and
respect ively. The rate constant for activation step in the mechanism is:
In the temperature range of 250 K to 450 K, the pre-exponent ial factor, A, for the reaction. Cl(g) + H2(g) → HCL(g) + H(g) is found to be equal to 1.20 x 1010 dm3 mol-1 s-1 . If M (Cl) = 35.453 g mol-1, M[H2] = 2.016 g mol-1, d(Cl) = 200 pm and d[H2] = 150 pm, the value of the Steric factor, P is:
The t1/2 of a reaction is doubled as the initial concentration of a reactant is increased 4 times. The order of the reaction is:
The relaxation time for the fast reaction:
and equilibrium constant is 1.0 x 10–3. The rate constant of the backward reaction is:
For which of the following reactions, the rate constant will be independent of ionic strength.
(I) H+ + Br– + H2O2
(II) C2H5COOCH3 + OH–
(III) S2O32– + I–
Which of the following plots is correct for an ionic reaction:
For the first order isomerization of an organic compound at 1300C, the activation energy is 108.4 KJ mol–1 and the rate constant is 9.12 x 10–4 s–1. The standard entropy of activation for this reaction is:
For reaction system,
2NO(g) + O2 (g) → 2 NO2 (g)
Volume is suddenly reduced to half of its value by increasing the pressure on it. If the reaction is of first order with respect to O2 and second order with respect to NO, the rate of reaction will.
Rate = K[A][B]; If the volume of reaction vessel is suddenly reduced 1/4th of initial value. The new rate will be effected by:
Under the same reaction conditions, initial concentration of 1.386 mol dm–3 of a substance becomes half in 40 s and 20 s through first order and zero order kinetics, respectively., Ratio of the rate constants for the first order k1 and zero order (k0) of the reaction is
T50 (Half-life period) of first-order reaction is 10 minute. Starting with 10 mol L–1. Rate after 20 minute is:
If the fermentation of sugar in an enzymatic solution that in 0.12 M. the concentration of the sugar is reduced to 0.06 M in 10 h and to 0.03 M in 20 h. What is the order of the reaction:
In a zero-order reaction for every 10° rise of temperature, the rate is doubled. If the temperature is increased from 10°C to 100°C, the rate of the reaction will become
|
83 videos|142 docs|67 tests
|