Test: Colligative Properties - 3 - Chemistry MCQ
25 Questions MCQ Test Physical Chemistry - Test: Colligative Properties - 3
The molal freezing point constant for water is 1.86 K kg mol–1. If 34.2 g of cane sugar (C12H22O11) is dissolved in 100 g of water, the solution will freeze at:
The ratio of the value of any colligative property for KCl solution to that for sugar is nearly ____ time:
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The vapour pressure of a solvent decreased by 10 mm Hg when a non-volatile solute was added to the solvent. The mole fraction of solute in solution is 0.2, what would be mole fraction of the solvent in decrease in vapour pressure is 20 mm of Hg:
Y g of non–volatile organic substance of molecular mass M is dissolved in 250 g benzene. Molal elevation constant of benzene is Kb. Elevation in its boiling point is given by:
The freezing point of aqueous solution contains 5% by mass urea, 1.0% by mass KCl and 10% by mass of glucose is
The values of observed and calculated molecular weights of silver nitrate are 92.64 and 170 respectively. The degree of dissociation of silver nitrate is:
Which of the following aqueous solutions has the highest boiling point & minimum freezing point?
Among the following the solution which shows the highest osmotic pressure at 25ºC is:
If a solute undergoes dimerisation and trimerisation, the minimum values of Vant Hoff factors respectively are:
Osmotic pressure of 4% (w/v) urea solution is 1.64 atm and that of 3.42% (w/v) cane sugar is 2.46 atm. When equal volume of the above two solutions are mixed, the osmotic pressure of the resulting solution is:
When mercuric iodide is added to an aqueous solution of potassium iodide then:
Which of the following will have same value of Vant Hoff factor (i) as that of K4[Fe(CN)6]:
If Pº and Ps are the V.P. of solvent and its solution respectively and N1 and N2 are the moles of solute and solvent then:
At constant temperature, the osmotic pressure of a solution is:
The value of Ebullioscopic constant or boiling point elevation constant depends on:
Which of the following pairs of solution are expected to be isotonic at the same temperature:
When a non-volatile solute is added to a pure solvent, which statement(s) hold true:
An aqueous solution of glucose boils at 100.01ºC. The mo lal elevat ion constant for water is 0.5 K Kg mol–1. The number of glucose molecules in the solution containing 100 g of water are x × 1021. The value of x is ______.
The apparent degree of ionization of KCl in water at 290 K is 0.86, the mass of KCl which must be made up to 1 dm3 of aqueous solution so as to produce the same osmotic pressure as the 4% solution of glucose at that temperature is ______g.
An aqueous solution containing 288 g of a non–volatile compound having the stoichiometric composit ion CxH2xOx in 90 g water boils at 101.24ºC at 1 atmospheric pressure. What is the value of x? (Given that: Kb(H2O) = 0.512 K Kg mol–1):
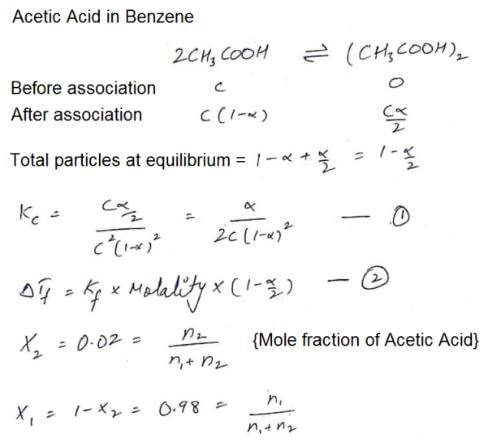
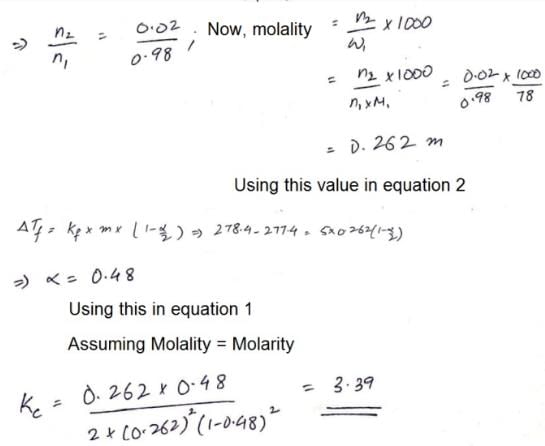
The freezing point of a solution of acetic acid (mole fraction is 0.02) in benzene is 277.4 K. Acetic acid exists partly as a dimer. Freezing point of benzene is 278.4 K and Kf for benzene is 5 K kg mol–1. The equilibrium constant for dimerisation is ______ kg mol–1:
The relative lowering of vapour pressure of an aqueous solution of a non–volatile solute of molecular weight 60 (which neither dissociates nor associates in the solution) is 0.018. If Kf of water is 1.86º cm–1, the depression in freezing point will be ______ºC.
|
83 videos|142 docs|67 tests
|