Test: Grignard Reagent - Chemistry MCQ
10 Questions MCQ Test Organic Chemistry - Test: Grignard Reagent
Which of the following reaction sequence that will best carry out the following preparation?

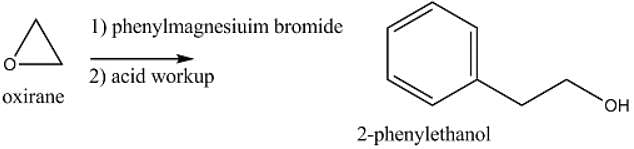
Which of the following reagents, when treated with phenylmagnesiuim bromide followed by acid workup, will yield 2-phenylethanol?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
A Grignard’s reagent may be made by reacting magnesium with which of the following compound?
Which of the following statements about Grignard reagent is false?
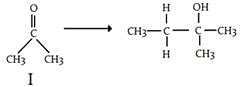
Which of the following compounds would not give tert-butyl alcohol when treated with excess methylmagnesium bromide?
Which of the following compounds gives a primary alcohol upon reaction with phenylmagnesium bromide?
Which of the following compounds gives a secondary alcohol upon reaction with methylmagnesium bromide?
Which of the following compounds does not give a tertiary alcohol upon reaction with methylmagnesium bromide/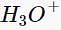 ?
?
Alkyl halides can be converted into Grignard reagents by _______________
|
35 videos|92 docs|46 tests
|