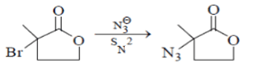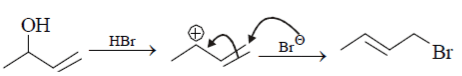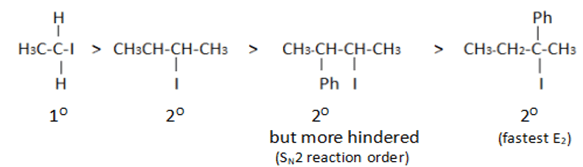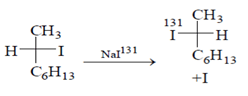Test: Nucleophilic Substitution Reaction - Chemistry MCQ
10 Questions MCQ Test Organic Chemistry - Test: Nucleophilic Substitution Reaction
Among the following reactions, which one is incorrect?
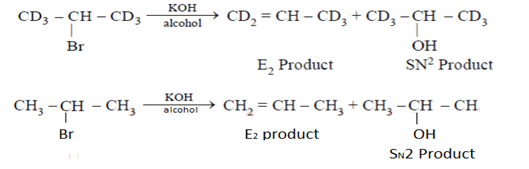
What will be the correct order of SN2/E2 ratio for the %yield of the product of the following halide?
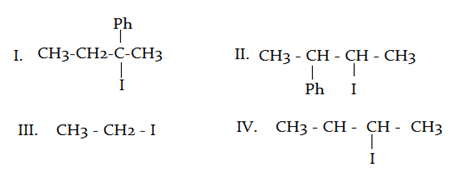
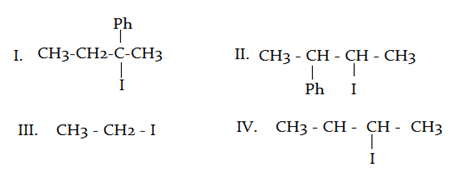
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
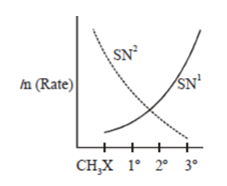
Which of the following curve correctly represents SN1 vs SN2?
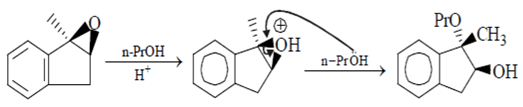
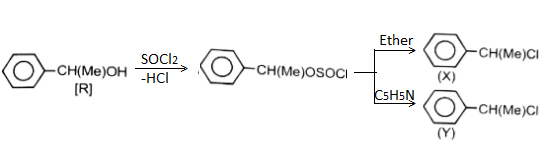
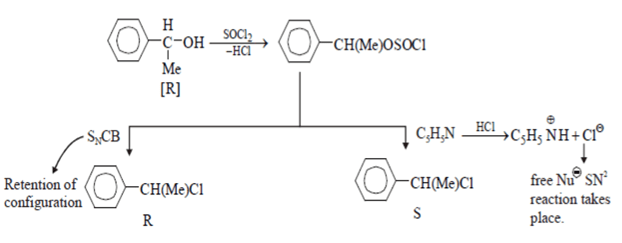
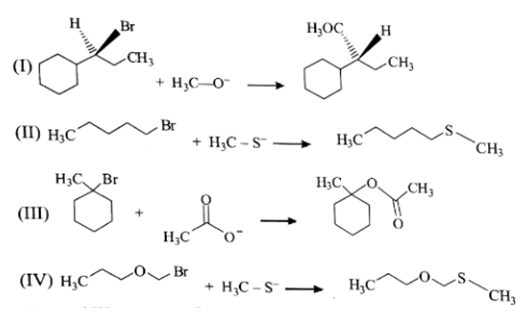
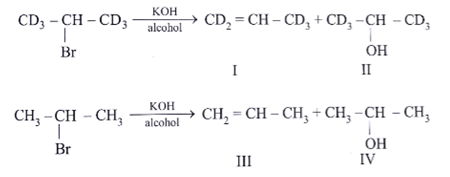
Which statement is incorrect about the following reaction?
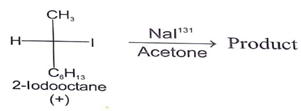
Which of the following reaction will go faster if the concentration of the nucleophile is raised?
Which of the following compound shows the correct decreasing order of solvolysis with aqueous ethanol?
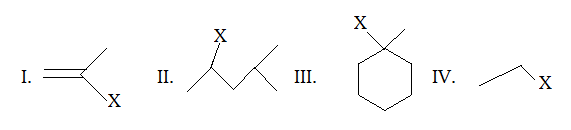
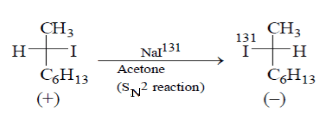
The correct choice is:
SN1 reaction undergoes through a carbocation intermediate as follows:
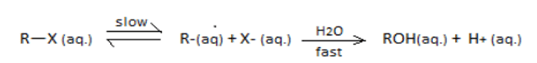
[R = t-Bu, iso-Pr, Et, Me] (X = Cl, Br, I)
The correct statements are:
I. The decreasing order of rate of SN1reaction is t-BuX > iso-PrX > EtX > MeX
II. The decreasing order of ionization energy is MeX > EtX > iso-PrX > t-BuX
III. The decreasing order of energy of activation is t-BuX > iso-PrX > EtX > MeX
Which one is an excellent substrate for SN2 reaction?
Which of the following is an incorrect statement?
|
35 videos|92 docs|46 tests
|