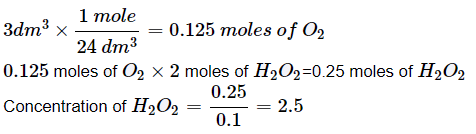Test: Mole Concept, Volumetric & Redox - 2 - Chemistry MCQ
30 Questions MCQ Test Physical Chemistry - Test: Mole Concept, Volumetric & Redox - 2
20 ml of xM HCl neutralizes completely 10 ml of 0.1M NaHCO3 and a further 5ml of 0.2M Na2CO3 solution to methyl orange end point. The value of x is:
The equivalent weight of Fe3O4 in the reaction Fe3O4 + KMnO4 → Fe2O3 + MnO2 would be
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
0.3g of an oxalate salt was dissolved in 100 ml solution. The solution required 90 ml of N/20 KMnO4 for complete oxidation. The % of oxalateion in salt is:
The number of moles of KMnO4 that will be needed to react with one mole of sulphite ion in acidic solution is:
A certain compound has the molecular formula X4O6. If 10g of compound contains 5.62g of x, the atomic mass of x is approx:
Starch paper is used to test for the presence of [NCERT 1979]
When a solution of NaOH and Na2CO3 is titrated against standard HCl, the end point due to phenolphthalein is obtained after the reaction:
Cu2+ + I– → Cu+ + I2, I2 + Na2S2O3 → NaI + Na2S4O6
If the moles of Cu2+ in above reaction consumed is ‘a’ then equivalents of Na2S4O6 will be:
In the above equation if moles required of S2O32– to titrate I2 was 2 then what were the moles of IO3– used:
IO3– + I– + H+ → I2 + H2O, I2 + S2O32– → I– + S4O62–
Then factor Of FeS2 in the following reaction FeS2 → Fe3+ + SO2 would be:
Fe0.94O → Fe3+, equivalent weight of reactants will be:
H3PO4 is a tribasic acid and one of its salt is NaH2PO4 . What volume of 1M NaOH solution should be added to 12g of NaH2PO4 to convert it into Na3PO4:
Sulphuryl chloride SO2Cl2, reacts with H2O to give mixture of H2SO4 and HCl. Aqueous solution of 1mole of SO2Cl2 will be neutralized by:
When Cr2O72– ion act as an oxidant in acidic medium, Cr3+ ion is formed. The number of moles of Sn2+ that are oxidized to Sn4+ by one mole of Cr2O72– ion would be:
A 0.518g sample of limestone is dissolved in HCl and then calcium is precipitated as CaC2O4. After filtering and washing the precipitate it requires 60 ml of 0.25N KMnO4 solution acidified with H2SO4 to titrate it as MnO4Θ + H+ + C2O42– → Mn2+ +CO2 +2H2O. The % of CaO in sample is:
Hydrogen peroxide in aqueous solution decomposes on warming to give oxygen acc. To the equation , 2H2O2 (aq.) → 2H2O (l) + O2 (g) . Under conditions where 1 mole of gas occupies 24dm3. 100cm3 of xM solution of H2O2 produces 3dm3 of O2. The x is:
What volume of O2 measured at standard conditions will be formed by the action of 100ml of 0.5N KMnO4 on H2O2 in an acid solution. The skeleton equation for the reaction is:
KMnO4 + H2SO4 + H2O2 → KHSO4 + MnSO4 + H2SO4 +H2O + O2
The Equivalent weight of the salt KHC2O4.H2C2O4.4H2O used as reducing agent is:
If the eq. weight of a compound ‘A’ is MA/4 when it reacts with compound ‘B’ whose eq. weight is MB/5 then 4 mole of ‘A’ requires:
Density of air is 0.001293 g/cc. Its vapour density is:
In which Mode of expression, the concentration of a solution remain independent of temperature
A gaseous oxide contains 30.4% of nitrogen, one molecule of which contains one nitrogen atom. The density of the oxide relative to oxygen is:
When KMnO4 acts as an oxidant and ultimately forms [MnO4]2–, MnO2, Mn2O3 and Mn2+, then number of electrons transferred in each case respectively is:
Which of the following is a redox reaction:
Which of the following reaction depicts the oxidizing behaviour of H2SO4:
In the standardization of Na2S2O3 using K2Cr2O7 by iodometry, the equivalent weight of K2Cr2O7 is:
are two isotopes of chlorine. If average atomic mass is 35.5 then ratio of these two isotopes is:
A gaseous mixture contains oxygen and nitrogen in the ratio of 1:4 by weight. Therefore, the ratio of their number of molecules is
|
83 videos|142 docs|67 tests
|