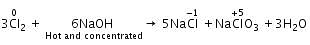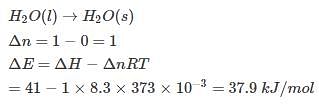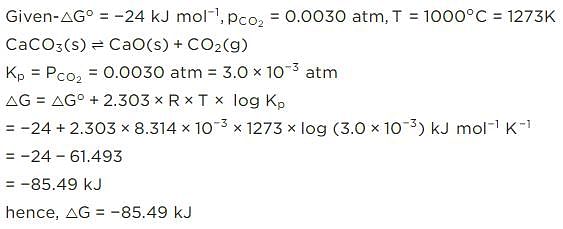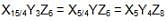UP PGT Chemistry Mock Test - 8 - UPTET MCQ
30 Questions MCQ Test UP PGT Mock Test Series 2024 - UP PGT Chemistry Mock Test - 8
If liquid A and B form ideal solution, than
[AIEEE-2003]
Which electrolyte will precipitate a negatively charged colloids (As2S3 sol) to a greater extend?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
When Cl2 gas reacts with hot and concentrated sodium hydroxide solution, the oxidation number of chlorine changes from
Which of the following sets of quantum numbers is correct for an electron in 4f orbital ? [AIEEE- 2004]
Lead emitted by vehicles interferes with development of:
Assuming that water vapour is an ideal gas, internal energy change (ΔE)when 1 mole of water is vaporised at 1 bar pressure and 100°C will be
(Given, molar enthalpy of vaporisation of water at 1 bar and 373 K = 41 kJ mol-1)
[IIT JEE 2007]
The curve showing the variation of adsorption with pressure at constant temperature is known as
A group of 14 element is converted into n – type semiconductor by dopping it with
For the cell (at 298 K)
Ag(s) | AgCl(s) | Cl-(aq) || AgNO3(aq) | Ag(s)
Which of the following is correct –
Consider the reactions
(A) H2O2 + 2HI → I2 +2H2O
(B) HOCl + H2O2 → H3O + Cl - - + O2
Which of the following statements is correct about H2O2 with reference to these reactions? Hydrogen perioxide is ________.
An aqueous solution contains 0.01 M RNH2 (Kb= 2 × 10-6) & 10-4 M NaOH.
The concentration of OH- is nearly:
Direction (Q. Nos. 17-20) This section contains 4 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THANT ONE is correct.
Q. Which of the following can be calculated based on Born-Haber cycle of formation of a lattice A+B- (s) from A(s) and 6 (g) ?
Comprehension Type
Direction (Q. Nos. 16-18) This section contains a paragraph, describing theory, experiments, data, etc.
Three questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage
Consider the following sequence of reaction,

Q.
The structure of compound B is
Passage III
A solution containing 0.684 g of cane sugar (C12H22011) in 100 g water freezes at - 0.037° C. A solution containing 0.585 g of NaCI in 100 g water freezes at - 0.342° C.
Q.
van’t Hoff factor (i) is
A coordination compound of cobalt has the molecular formula containing five ammonia molecules, one nitro group and two chlorine atoms for one cobalt atom. One mole of this compound produces three ions in an aqueous solution. The aqueous solution on treatment with an excess of AgNO3 gives two moles of AgCI as a precipitate. The formula of the complex and the isomerism shown by this is
Which of the following metal solution cannot be prepared by Bredig’s arc method?
An electrochemical cell was based on the following reaction:
Mn(OH)2(s) + H2O2(aq) → MnO2(s) + 2H2O (e)
During the opeartion of this for 1 min, 0.135 g of MnO2 was produced. What is the average electric current (in ampere ) produced by the cell?
From the following, pick out the potential energy profile for a SN1 reaction.
Solid X is a very hard electrical insulator in solid as well as in molten state. It melts at extremely high temperature. Solid X is a
Kc forthe decomposition of NH4HS(s) is 1.8x 10-4 at 25°C.
If the system already contains [NH3] = 0.020 M, then when equilibrium is reached, molar concentration are
For the reaction, at 1000° C
ΔG° = - 24 kJ mol-1, 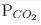 = 0.0030 atm.
= 0.0030 atm.
Q. Hence, ΔG at this temperature is
A gas absorbs a photon of 355 nm and emits at two wavelengths. If one of the emissions is at 680 nm, the other is at:
This process in which nutrient enriched water bodies support a dense plant population, which kills animal life by depriving it of oxygen and results in subsequent loss of biodiversity, is called
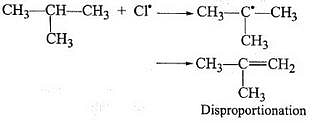
During free radical bromination of isobutane, an alkene is produced as by product via disproportionation of the intermediate alkyl free radical. What is this alkene?
Coordination numbers in a square close packed structure and hexagonal close packed structure respectively, are
A crystal is made of particle X, Y & Z. X forms FCC packing, Y occupies all octahedral voids of X and Z occupies all tetrahedral voids of X, if all the particles along one body diagonal are removed then the formula of the crystal would be –
Which one of the following exists in the oxidation state other than +3?
|
30 tests
|