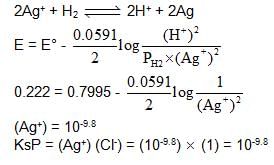HPSC PGT Chemistry Mock Test - 3 - HPSC TGT/PGT MCQ
30 Questions MCQ Test HPSC PGT Mock Test Series 2024 - HPSC PGT Chemistry Mock Test - 3
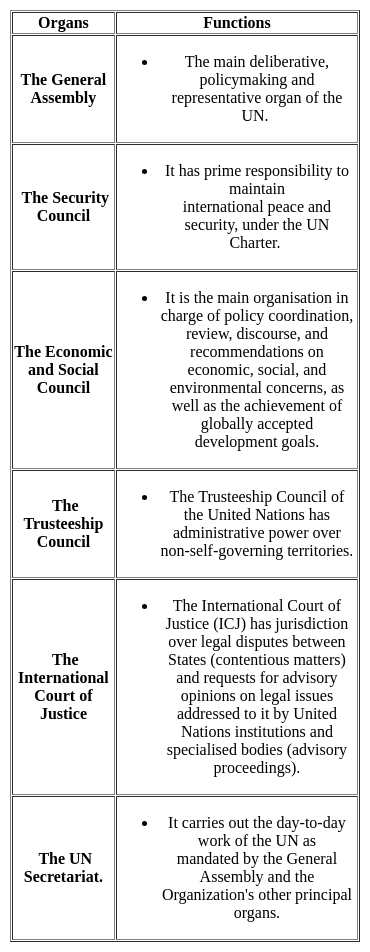
How many main organs are there in United Nations Organization?
Who among the following has been appointed as the first ADGP Traffic of Haryana recently?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
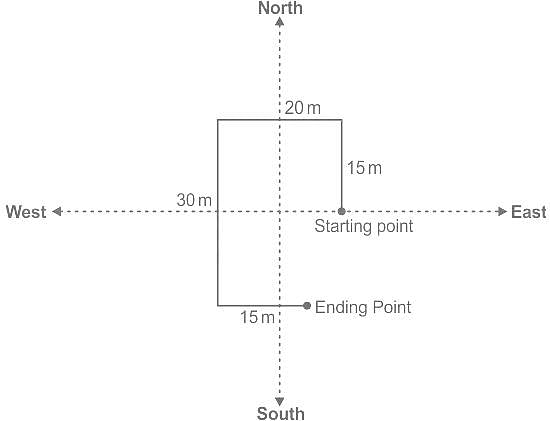
Ramu walks 15m towards the north then turns left and covers 20m then covers also 30m by turning south then again turns left and covers 15m. What is the total distance covered by him?
Under NEP 2020, what is the target for the Gross Enrollment Ratio (GER) in higher education by 2035?
One kg of carbon produces __________ kg of carbon dioxide.
The oxidation number of an element in a compound is evaluated on the basis of certain rules. Which of the following rules is not correct in this respect?
Which of the following elements has the largest ionisation enthalpy?
During dehydration of alcohols to alkenes by heating with conc. H2SO4 the initiation step is-
[AIEEE-2003]
Which of the following can exhibit linkage isomerism?
In which of the following boiling point of Column I is not higher than that of Column II?
Wave number of a spectral line for a given transition is x cm-1 for He+, then its value for Be3+ (isoelectronic of He+)for the same transition is
Yellow colour of NaCl crystals in sodium vapour is due to
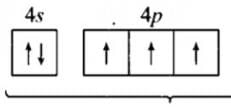
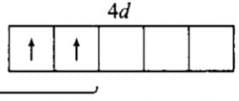
A sub-shell with n = 6 , l = 2 can accommodate a maximum of
On moving down a group the number of valence electrons:
According to Bohr's theory, the angular momentum of an electron in 5th orbit is - [AIEEE 2006]
Among the following statements the one that is not true about Mendeleev’s Periodic Table is:
Ferric hydroxide is a negative sol, which of the following electrolyte will coagulate it most:
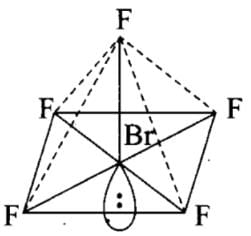
Which of the following has a square pyramidal shape?
Unleaded paint and petrol were introduced because:
Primary alcohol can easily be prepared from primary alkyl halide via SN2 reaction with aqueous NaOH. However, similar method does not work for the preparation of tertiary alcohol. Which reaction can be used for the efficient preparation of tertiary alcohol {tertiary butanol) from tertiary butyl bromide?
If Ksp for HgSO4 is 6.4 × 10-5, then solubility of this substance in mole per m3 is
How many stereoisomers exist for the compound 4-(1- propenyl) cyclohexane ?
Pick out the most reactive alkyl halide for an SN1 reaction.
Following cell has EMF 0.7995 V.
Pt | H2 (1 atm) | HNO3 (1M) || AgNO3 (1M) | Ag
If we add enough KCl to the Ag cell so that the final Cl- is 1M. Now the measured emf of the cell is 0.222 V.
The Ksp of AgCl would be :
In which reaction a chiral reactant is giving a chiral product