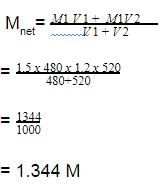HPSC PGT Chemistry Mock Test - 4 - HPSC TGT/PGT MCQ
30 Questions MCQ Test HPSC PGT Mock Test Series 2024 - HPSC PGT Chemistry Mock Test - 4
Who is the first women IPS to become the police Commissioner of Gurugram in Feb 2022?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Neeraj Chopra, a sportsperson from Haryana is associaed with which of the following sports?
The rate is independent of the concentration of the reactants in
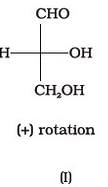
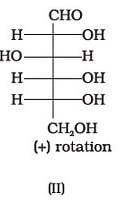
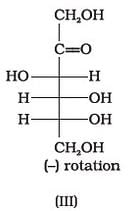
Optical rotations of some compounds along with their structures are given below which of them have D configuration.
A gas absorbs 200 J of heat and expands by 500 cm3 against a constant pressure of 2 x 105 Nm-2. Change in internal energy is
Two solutions of a substance (non electrolyte) are mixed in the following manner. 480 ml of 1.5 M first solution + 520 mL of 1.2 M second solution. What is the molarity of the final mixture ?
[AIEEE-2005]
Which of the following electrodes will act as anodes, when connected to Standard Hydrogen Electrode?
The catalytic activity of transition metals and their compounds is mainly due to
Mono-chloro product (inculding steroisomers)are :
The adsorbent used in decolouration of vinegar and sugar solution is
Tendency of an atom in a molecule to attract the shared pair of electron towards itself is called:
What is aggregation of colloidal particles into insoluble precipitate by addition of some suitable electrolyte called?
Which substance is added to water containing suspended impurities to coagulate the suspended impurities and make water fit for drinking purposes.
One of the electrons of the highest energy level is taken to next excited state in the following diatomic species. Select the species which undergoes change in bond order?
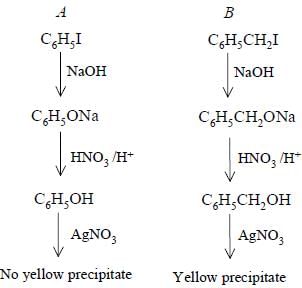
Bottles containing C6H5I and C6H5CH2I lost their original labels. They were labelled A and B for testing. A and B were separately taken in test tubes and boiled with NaOH solution. The end solution in each tube was made acidic with dilute HNO3 and then some AgNO3 solution was added. Substance B gave a yellow precipitate.
Which one of the following statements is true for this experiment ?
[AIEEE-2003]

Suitable reagent for following cenversion will be :
How many different diols product are formed in the following reaction?
The correct statement regarding elements of symmetry and chirality of compound is
Consider the following oxidation/reduction process,
Q. Magnetic moment does not change in
An aqueous solution is 34% H3P04 by mass and has density 1.209 g mL -1 Molarity (I), molality (II) and normality (III) respectively, are
Passage II
A solution containing 10 g of a dibasic acid in 1000 g of water freezes at -0.15° C. 10 mL of the acid is neutralised by 12 mL of 0.1 N NaOH solution. [Kf (H20 ) = 1.86° mol-1 kg]
Q.
van’t Hoff factor (/') of the dibasic acid is
what is incorrect regarding cis -1, 3-dibromo - trans-2,4-dichlorocyclobutane ?
Passing carbon dioxide through slaked lime gives: