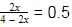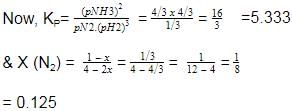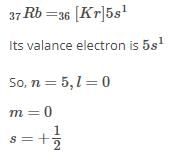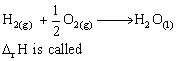HPSC PGT Chemistry Mock Test - 6 - HPSC TGT/PGT MCQ
30 Questions MCQ Test HPSC PGT Mock Test Series 2024 - HPSC PGT Chemistry Mock Test - 6
Mahatma Gandhi was first arrested in which railway station of Haryana?
During ancient times, Mahendragarh town of this state was known as
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
A word with letters jumbled has been given. Choose the correct order of letters which are required to form the correct word.
Jumbled word: PTIOISCL
If you are planning to conduct an exam particularly for the backward children, then the exam would be
Which of the following skills is associated with emotional intelligence?
Consider the ground state of Cr atom (Z = 24). The numbers of electrons with the azimuthal quantum numbers, l = 1 and 2 are, respectively [AIEEE- 2004]
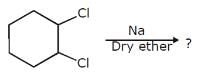
What is the order of SN2 reaction of the alkyl halide?
The correct order of thermal stability of the hydrides of group 16 elements is
A complex compound in which the oxidation number of a metal is zero is
Thermal decomposition of a compound is of first order. If 50% of a sample of a compound is decomposed in 120 min, the time taken for 90% completion is
Which of the following correctly describe the relative nucleophilicities of methoxide and tertiary butoxide ion?
Calculate resonance energy of N2O from the following data
ΔfH° (N2O) = 82 kJ mol-1
Which of the following forms a colloidal solution in water?
[Ti (H2O)6]3+ absorbs green and yellow region part of visible light. Then the transmitted colour of the compound is
For the reaction,
2SO2 (g) + O2 (g) 2SO3 (g) + 188.3 KJ
2SO3 (g) + 188.3 KJ
the number of moles of SO3 formed is increased if
Sodium pentacyanonitrosylferrate(II) is also called?
The metabolism of hormones in human body is an example of
Which of the following equation depicts reducing nature of H2O2?
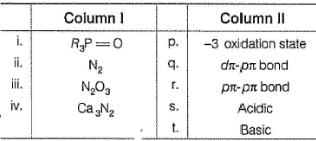
Match the Column I with Column II and mark the correct option from the codes given below :
At 700 K and 350 bar, a 1 : 3 mixture of N2(g) and H2(g) reacts to form an equilibrium mixture containing X (NH3)= 0.50. Assuming ideal behaviour Kp for the equilibrium reaction,
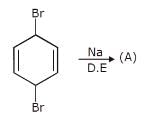
Which of the alkyl chlorides listed below undergoes dehydrohaiogenation in the presence of a strong base to give 2-pentene as the only alkene product?
Among the following sets, highest boiling points are of the species.
I. HF, HCI, HBr, HI
II. H2O, H2S, H2Se, H2Te
III. NH3,PH3, AsH3,SbH3
The correct set of four quantum numbers for the valence electron of rubidium atom (Z = 37) is
[JEE Main 2013]
Compounds which have same molecular formula but different structural formula is called
Direction (Q. Nos. 11-14) This section contains 2 paragraph, wach describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given options (a),(b),(c),(d).
Passage l
Sulphur undergoes a phase transition between 80 and 110°C
S(rhombic) S (monoclinic); ΔH° = 3.213 kJ mol-1; ΔS° = 8.71 JK-1 mol-1
Q. Select the correct alternate(s).
The constant k used in rate equation is known as