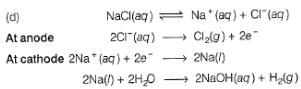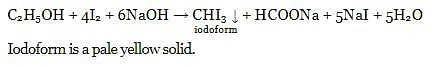HPSC PGT Chemistry Mock Test - 7 - HPSC TGT/PGT MCQ
30 Questions MCQ Test HPSC PGT Mock Test Series 2024 - HPSC PGT Chemistry Mock Test - 7
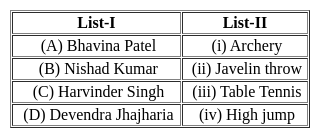
Match list-I with list-II and identify the correct answer from the code given below –
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Select the word-pair in which the two words are related in the same way as the two words in the following word-pair.
International Yoga Day : 21st June :: ? : ?
After class 10, Ankit is confused about the subject he should opt for in class 11. His confusion could be solved by taking
You have been coming across children who have very limited or no concentration in their tasks. They do not show any interest in communicating with others and are quite insistent by nature. These children are stressed children who can be identified by a teacher when they show
Which of the following are the examples of strong nucleophiles but weak base in protic solvents?
I. CH3S-
II. CH3O-
III. I-
IV. H2O
V. F-
The complex [Fe(H2O)5NO]2+ is formed in the ring-test for nitrate ion when freshly prepared FeSO4 solution is added to aqueous solution of
followed by the addition of conc. H2SO4. NO exists as NO+ (nitrosyl).
Q. Magnetic moment of Fe in the ring is
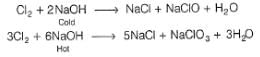
What does hydrogen peroxide liberate from potassium iodide?
At 298K the standard free energy of formation of H2O(L) is – 237.20kJ/mole while that of its ionisation into H+ ion and hydroxyl ions is 80 kJ/mole, then the emf of the following cell at 298 K will be
H2(g,1 bar) | H+ (1M) || OH– (1M) | O2 (g, 1bar)
What product are formed during the electrolysis of a concentrated aqueous solution of sodium chloride using an electrolytic cell in which electrodes are separated by a porous pot?
I. Cl2(g)
II. NaOH(aq)
III. H2(g)
IV. NaClO(aq)
V. NaClO3(aq)
Select the correct choice.
Nucleic acids are the polymers of ______________.
Direction (Q. Nos. 7 and 14) This section contains 8 questions. when worked out will result in an integer from 0 to 9 (both inclusive)
Q.
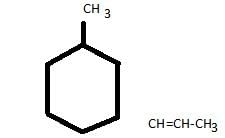
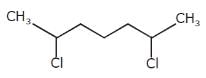
How many stereoisomers exist in the compound 1-methyl-3-(1-propenyl) cyclohexane ?
Which of the following structures represents a chiral compound ?
The major organic product formed in the following reaction is
The salt which finds uses in qualitative inorganic analysis is -?
In the following conversion of chromite into soluble chromium salt
4Fe(CrO2)2+ 8Na2CO3 + 7O2 --> 2Fe2O3 + 8Na2CrO4 + 8CO2
There is
Direction (Q. Nos. 7 - 10) This section contains 4 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Q. Predict product(s) in the following reaction.
Provide the structure of the major organic product given in the following reaction.
Among the alkenes which one produces tertiary butyl alcohol on acid hydration
The standard enthalpies of formation of CO2(g), H2O (l) and glucose (s) at 25°C are - 400 kJ mol-1, - 300 kJ mol-1 and - 1300 kJ mol-1 respectively. The standard enthalpy of combustion per gram of glucose at 25°C is
[JEE Advanced 2013]
Which method cannot be used for the coagulation of the lyophobic sol?
The primary difference between the modern periodic table and Mendeleev's periodic table is:
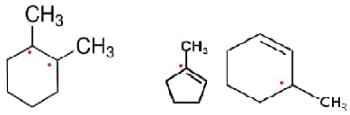
How many assymmetric carbon atoms are present in
(i) 2-Dimethyl cyclohexane
(ii) 3-Methyl cyclopentene
(iii) 3-Methylcyclohexene
Atmosphere traps the sun’s heat near the earth’s surface. This is called:


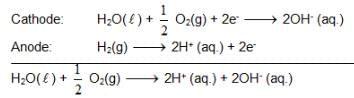 also we have
also we have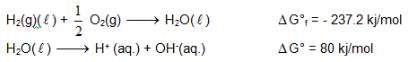 Hence for cell reaction
Hence for cell reaction