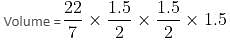DSSSB PGT Chemistry Mock Test - 3 - DSSSB TGT/PGT/PRT MCQ
30 Questions MCQ Test DSSSB PGT Mock Test Series 2024 - DSSSB PGT Chemistry Mock Test - 3
Directions: Study the following information carefully and answer the questions given below:
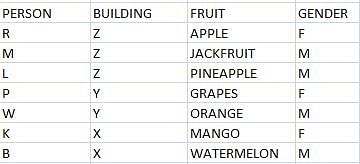
1) Seven friends R, M, K, P, L, W, and B live in three different buildings, i.e. X, Y, and Z. Not less than two or more than three live in any of the buildings.
2) Each of them has a liking for different fruits among apple, jackfruit, watermelon, orange, grapes, pineapple, and mango, not necessarily in that order.
3) Three among them are girls, one each every building. W likes orange and stays in building Y with P only. M lives in building Z and he likes jackfruit.
4) None in building X likes apple or grapes.
5) R and L do not stay in building X.
6) K is R’s close friend and she does not like watermelon. L likes pineapple.
7) None of the girls like orange and one of them likes apple. R does not like grapes.
Q.Who likes mango?
Two less than 5 times a number is greater than the third multiple of the number. So the number must be
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Directive Principles of State Policy are based on the principles of:
Direction: While the modern India has made progress in various fields, the priceless intangible heritage of works like the Sama Veda have gone unnoticed-a great loss indeed. We have, it could be said, mindlessly pursed a line of development causing
unwelcome changes to the very fabric of our culture; not very different from iconoclastic behavior. In spite of the fact that a large number of rich Indians possess more wealth than the world's richest, we demonstrate such ignorance about the Vedas in general and Sama Veda in particular. One needs to find out why the world’s oldest composite literature on religion, sciences, humanities and spirituality attracts little public attention today. The yajnas, apart from their religious functions, were unfortunately termed as mere ‘rituals’ even by Indian scholars and so their secular significance has been undermined. It has been shown in a premiere scientific institute in Pune that the Yajna bhasma being of the size of nano-particles had positive effects on human health and the environment.
Q. Which of the following is having positive effects on human body ?
If the diameter of a cylindrical drum is 1.5 m and the height is 1.5 m, then maximum how many smaller aluminium cylindrical cans can be completely filled by the liquid in the cylindrical drum, if the radius and height of each can are 0.2 metres and 0.4 metres, respectively?
निर्देश: निम्नलिखित गद्यांश को पढ़कर निम्न प्रश्न का उत्तर दें।
संसार में सबसे मूल्यावान वस्तु समय है क्योंकि दुनिया की अधिकांश वस्तुओं को घटाया-बढ़ाया जा सकता है, पर समय का एक क्षण भी बढ़ा पाना व्यक्ति के बस में नहीं है। समय के बीत जाने पर व्यक्ति के पास पछतावे के अलावा कुछ नहीं होता। विद्यार्थी के लिए तो समय का और भी अधिक महत्त्व है। विद्यार्थी जीवन का उद्देश्य है शिक्षा प्राप्त करना। समय के उपयोग से ही शिक्षा प्राप्त की जा सकती है। जो विद्यार्थी अपना बहुमूल्य समय खेल-कूद, मौज-मस्ती तथा आलस्य में खो देते हैं वे जीवन भर पछताते रहते हैं, क्योंकि वे अच्छी शिक्षा प्राप्त करने से वंचित रह जाते हैं और जीवन में उन्नति नहीं कर पाते। मनुष्य का कर्तव्य है कि जो क्षण बीत गए हैं, उनकी चिंता करने के बजाय जो अब हमारे सामने हैं, उसका सदुपयोग करें।
Q. उपर्युक्त गद्यांश के अनुसार संसार में सबसे मूल्यवान वस्तु क्या है?
How many atoms of hydrogen are in 67.2 L of H2 at STP?
Packing efficiency (%) of different types of unit cells is given. Select the correct packing efficiency.
Emission spectrum of a material results from the material's (atom or molecules)
Which among the following substances is an example of multimolecular colloids?
The simplest way to check whether a system is a colloid is by using:
If liquids A and B form an ideal solution _
[AIEEE-2003]
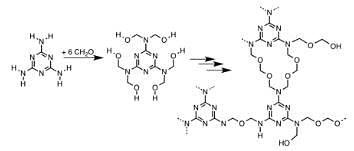
Lewis structure of the elements M and Z are shown below.
Compound formed is
Different kinds of bonds and interaction present within CuSO4 • 5H2O. They can be
I. σ-bond
II. π-bond
III. coordinate bond
IV. electrostatic force of attraction
V. H-bond due to dipole-dipole interaction
VI. H-bond due to ion-dipole interaction
Select the correct types of bonds/interactions.
The species having pyramidal shape is
[IIT JEE 2010]
The physical properties of isotopes differ due to:
The actinoids Exhibit more member of oxidation states in general than the lanthanoids. This is because
Which of the following does not show optical isomerism?
In the given sequence reaction which of the following is the correct structure of compounds A.
What is true regarding a meso form of a compound?
Which of the following on reaction with excess of NaHSO3 in aqueous solution will give mixture of salts which can be separated into two fractions by fractional crystallisation?
Which is the best hydride (H-) donor in the key step of Cannizaro reaction?
Which one of the following is a water soluble vitamin?