EMRS PGT Chemistry Mock Test - 1 - EMRS MCQ
30 Questions MCQ Test EMRS PGT Mock Test Series 2024 - EMRS PGT Chemistry Mock Test - 1
What will be the color of litmus solution when mixed with sulfuric acid?
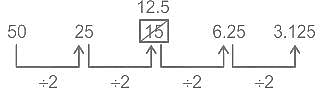
Find the wrong term in the given series?
50, 25, 15, 6.25, 3.125
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
A word with letters jumbled has been given. Choose the correct order of letters which are required to form the correct word.
Jumbled word: PTIOISCL
How many '8' are followed by even number and preceded by an odd number?
184381483287848568784186
Direction: Read the following information carefully and answer the questions that follow.
A blacksmith has five iron articles A, B, C, D and E each having a different weight.
I. A weight is twice as much as of B.
II. B weight is four and half times as much as of C.
III. C weight is half times as much as of D.
IV. D weight is half as much as of E.
V. E weight is less than A but more than C.
Q. Which of the following represents the descending order of weights of the articles?
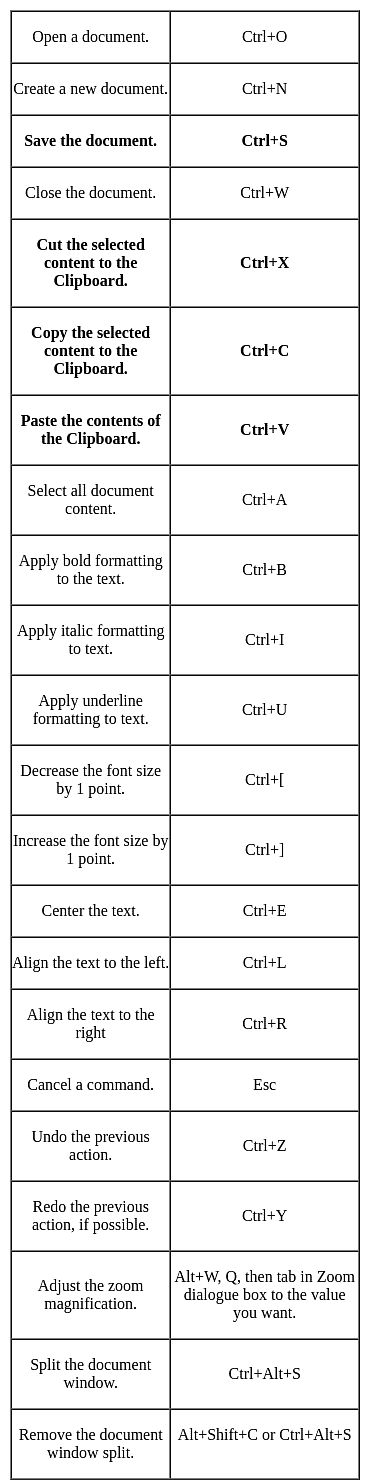
In MS-Word and MS-Excel, which of the following is the shortcut key to paste content to /from clipboard:
The greatest cause for failure of a new teacher can be found in the area of
The basis of selection of a teaching aid is
- age of the learner
- objectives of teaching
- intellectual level of the learner
Choose from the options given below:
Which property of colloids is applied in rubber plating & sewage disposal?
Only One Option Correct Type
This section contains 16 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct
Q.
During the electrolysis of aqueous Zn(NO3)2 solution
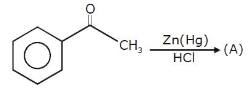
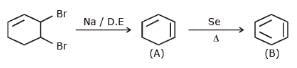
For the following reactions sequence
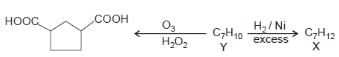
The structure consistent with X and Y are:
One mole of N2O4(g) at 300 K is left in a closed container under one atm. It is heated to 600 K when 20% by mass of N2O4 (g) decomposes to NO2(g). The resultant pressure is :
An atom has a mass of 0.02 kg & uncertainity in its velocity is 9.218 × 10-6 m/s then uncertainity in position is (h = 6.626 × 10-34 J - s) [AIEEE- 2002]
Only One Option Correct Type
Direction (Q. Nos. 1-8) This section contains 8 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q.
The incorrect statement concerning E1 reaction is
Due to the presence of electrons in the inner shells, the electron in the outer shell will not experience the full positive charge of the nucleus (Ze). This is known as
Which one of the following is not a colligative property?
The atom which defines the structure of a family of organic compounds and their properties is called ___________
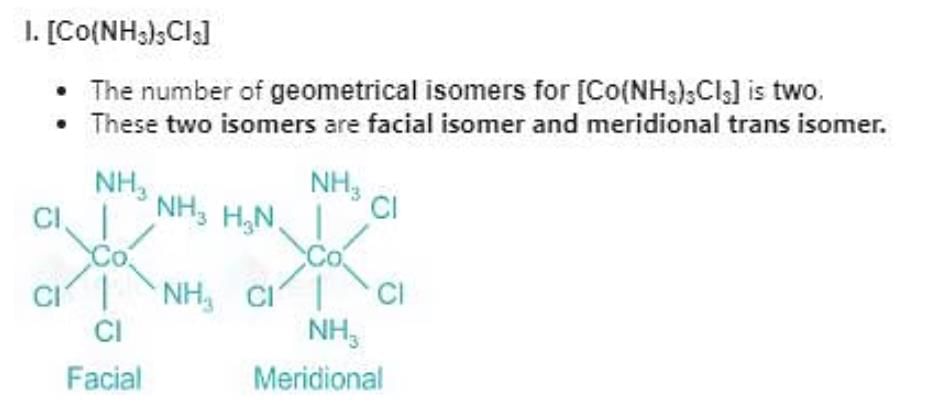
Which of the following compounds will show facial and meridional isomerism?
(A)[Co(NH3)3Cl3]
(B)[Co(acac)3]
(C)[Co(dien)(NO2)3]
(D)[Co(gly)3]
The following three aspects of intelligence are dealt with by Sternberg's triarchic theory except:
In the following question, out of the four alternatives, select the word similar in meaning to the word given.
Slander
|
8 docs|45 tests
|