EMRS PGT Chemistry Mock Test - 2 - EMRS MCQ
30 Questions MCQ Test EMRS PGT Mock Test Series 2024 - EMRS PGT Chemistry Mock Test - 2
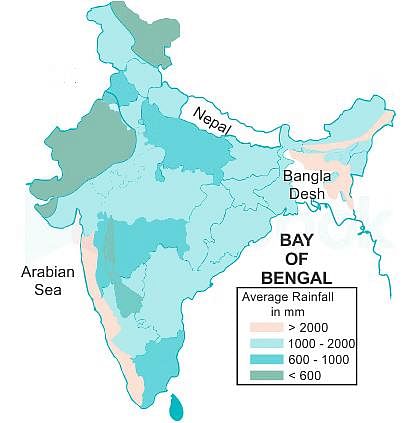
Which one of the following places receives the highest rainfall in the world?
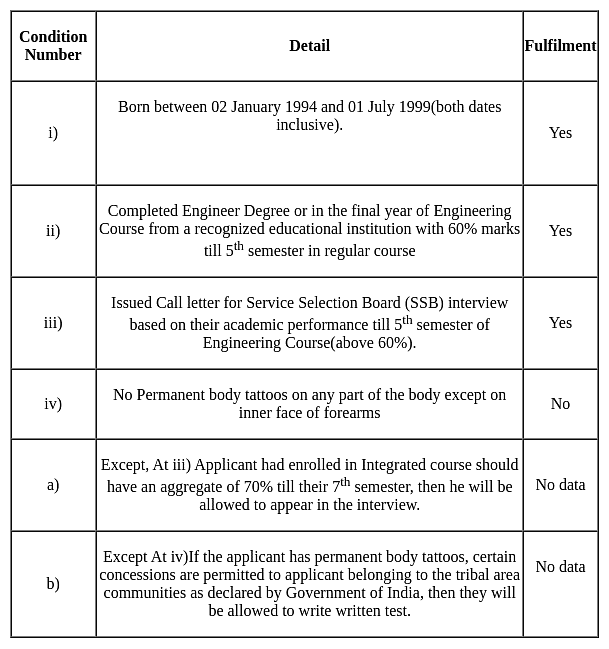
Vishnu born on 15 January 1995, completed his engineering degree, with 85% marks till 5th semester in regular course, he has a permanent body tattoo in his shoulder.
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
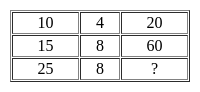
Select the number which can be placed at the sign of the question mark (?) from the given alternatives.
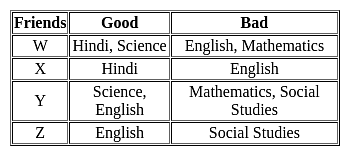
Which of the following friends are good in English, Mathematics, and Science but poor in Social Studies?
What is important in ICT supported teaching learning strategies?
The role of ICT as a support and learning school management does not include
Directions: In the following question, a statement of Assertion is given, followed by a corresponding statement of Reason just below it. Of the statements, mark the correct answer from the options given below.
Assertion (A): Child who faces difficulties in teaching-learning process is considered as a child with learning disability.
Reason (R): The child affected with dyscalculia asks the person to repeat questions asked again and again.
In order to help a mentally challenged child in your class, which of the following strategies would you adopt?
At 25 ° C, the density of 18 MH2SO4 is 1.8 g cm3. Thus, mass percentage of H2SO4 in aqueous solution is
Maximum number of electrons in a subshell with l = 3 and n = 4 is
Out of NH3, H2O and HF, which has the highest magnitude of Hydrogen bonding:
The ratio of bond pairs and lone pairs in a P4 molecule is
In oxygen difluoride (OF2) and dioxygen difluoride(O2F2), the oxygen is assigned an oxidation number of
Order of the photochemical reaction occurring between hydrogen and chlorine is
The decreasing order of the repulsive interactions between various electron pairs is:
Which of the following types of octahedral complexes will exhibit geometrical isomerism (where, M = metal, a, b = achiral ligands)?
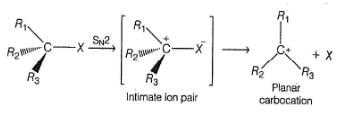
In SN1 reaction, racemisation occurs if the reaction occurs at a stereogenic centre, however, 50:50 mixture of enantiomers are rarely obtained, why?
A first order reaction is 50% completed in 1.26 × 1014 s. How much time would it take for 100% completion?
For which of the following reaction the units of rate constant and rate of the reaction are same?
If a is the degree of dissociation of Na2SO4, the vant Hoff's factor (i) used for calculating the molecular mass is–
[AIEEE-2005]
Which of the following represents the decreasing order of van der Waals’ forces in halogens ?
Passage
Phosphorus was discovered by Brand (1669), Scheele isolated from bone ash and Lavoisier proved its elemental nature (1777). The principal minerals are phosphate rock, fluoroacetate, and chloroacetate. Phosphorus is prepared by the direct reduction of phosphorite by carbon in the presence of silica. It exists in different allotropic forms such as yellow or white, red, a-black,f3-black, etc. White P is most reactive, poisonous, glows in dark, and readily catches fire due to unstable discrete P4 molecules. Red P is inert, non-poisonous, does not glow, etc., due to its polymeric structure. a-black, f3 -black allotropes are also chemically inert, do not ignite at normal temperature. It has a layer structure like graphite and acts as a conductor.
Q. The allotrope of phosphorus with low ignition temperature is:
Improve the bracketed part of the sentence with the parts given below.
Q. It is a known fact that some people have (blind faith) in superistitions in our country.
In the following question, out of the four alternatives, select the word similar in meaning to the word given.
Rumble
The question below consists of a set of labeled sentences. Out of the four options given, select the most logical order of the sentences to form a coherent paragraph.
P: They also went for dinner to an Italian restaurant after the movie.
Q: The bond between them still had been the same though.
R: They had been away for some time and were meeting after a gap.
S: The boys went out for a movie on Friday night.
|
8 docs|45 tests
|