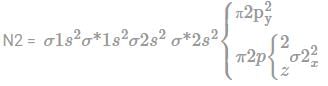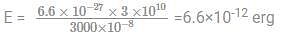Test: CSIR-NET Chemical Sciences Mock Test - 4 - UGC NET MCQ
30 Questions MCQ Test CSIR NET Exam Mock Test Series 2024 - Test: CSIR-NET Chemical Sciences Mock Test - 4
A and B invest in a business in the ratio 3 : 2. If 5% of the total profit goes to charity and A's share is Rs. 855, the total profit is :
If each edge of a cube is increased by 50%, find the percentage increase in its surface area.
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
A is B's sister. C is B's mother. D is C's father. E is D's mother. Then, how is A related to D?
Fresh fruit contains 68% water and dry fruit contains 20% water. How much dry fruit can be obtained from 100 kg of fresh fruits ?
A student multiplied a number by 3/5 instead of 5/3, What is the percentage error in the calculation ?
In an election between two candidates, one got 55% of the total valid votes, 20% of the votes were invalid. If the total number of votes was 7500, the number of valid votes that the other candidate got, was :
If 20% of a = b, then b% of 20 is the same as :
The dimensions of a floor are 18 × 24 What is the smallest number of identical square tiles that pave the entire floor without the need to break any tile?
How many digits are there in 316 when it is expressed in the decimal form?
⊕ and ⊙ are two operators on numbers p and q such that p⊕q= If x ⊕ y = 2 ⊙ 2, then x =
If x ⊕ y = 2 ⊙ 2, then x =
Fill in the blank: F2, ________, D8, C16, B32, A64.
Comparing numerical values, which of the following is different from the rest?
The product of the perimeter of a triangle, the radius of its in - circle, and a number gives the area of the triangle. The number is
AB and CD are two chords of a circle subtending 60° and 120° respectively at the same point on the circumference of the circle. Then AB : CD is
A man buys alcohol at Rs. 75/L, adds water and sells it at Rs. 75/L making a profit of 50%. What is the ratio of alcohol to water?
The statement that describes C60 is:
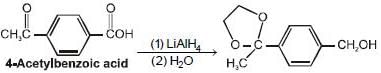
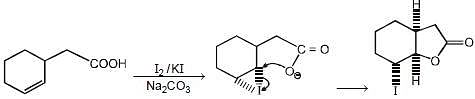
The major product formed in the following reaction
The major product formed in the reaction given below is

The number of possible isomers for [Ru(bpy)2Cl2] is (bpy = 2,2'-bipyridine)
One litre of gas A at two atmospheric pressure and two litres of gas B at three atmospheric pressure are mixed in a four -litre flask to form an ideal gas mixture. What will be the final pressure of the gaseous mixture if the gases initially and finally where at the same temperature?
Which of the following reactions does not produce an alkyl halide?
60 grams of gaseous C2 H6 are mixed with 28 grams of gaseous carbon monoxide. The pressure of the resulting gaseous mixture is 3 atm. The partial pressure of C2 H6 in the mixture is
Alcohols do not show which of the characteristics?
The product formed in the following rearrangement reaction is —

The highest occupied MO in N2 and O2 + respectively are (take x-axis as internuclear axis)
The intermediate Y and the product Z in the following reaction are respectively —

Given that Plank’s constant = 6.6 × 10–27 erg - second, velocity of light = 3 ×1010 cm/s, the energy of a photon of wavelength 3000 Å will be
In the following four elements, the ionization potential of which one is the highest ?
The Nernst equation,
E = E° -(RT/nF) nQ
indicates that the equilibrium constant HC will be equal to Q when
The possible values of total angular momentum resulting from the additions of angular momentum with quantum numbers.
j1 = 2, j2 = 4


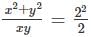




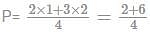 =2 atmosphere
=2 atmosphere