Test: CSIR-NET Chemical Sciences Mock Test - 6 - UGC NET MCQ
30 Questions MCQ Test CSIR NET Exam Mock Test Series 2024 - Test: CSIR-NET Chemical Sciences Mock Test - 6
Salaries of Ravi and Sumit are in the ratio 2:3. If the salary of each is increased by Rs. 4000, the new ratio becomes 40:57. What is Sumit's salary?
The L.C.M of two numbers is 495 and their H.C.F is 5. If the sum of the numbers is 100, then their difference is
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
A, B and C enter into a partnership and their shares are in the ratio 1/2 : 1/3 : 1/4. After 2 months, A withdraws half of his capital and after 10 months, a profit of Rs. 378 is divided among them. What is B's share ?
A sum of money lent at compound interest for 2 years at 20% per annum would fetch Rs.482 more, if the interest was payable half yearly than if it was payable annually . The sum is
The average of 20 numbers is zero. Of them, at the most, how many may be greater than zero ?
What least number must be subtracted from 13601, so that the remainder is divisible by 87 ?
405 sweets were distributed equally among children in such a way that the number of sweets received by each child is 20% of the total number of children. How many sweets did each child recieve ?
The ratio of the outer and the inner circumferences of a circular path is 11 ∶ 7. If path is 20 metres wide, then what is the radius of the inner circle?
A dishonest shopkeeper professes to sell pulses at the cost price, but he uses a false weight of 950gm for a kg. Find his gain percent.
In grade 11 of a school, 40 students opted for Physics, 17 opted for Biology, and 20 opted for Chemistry. If the total number of students in grade 11 was 60, all of these students opted for at least one of the three subjects mentioned here, and exactly five of these students opted for all these three subjects, what is the probability that a randomly selected student of grade 11 of this school would have opted for exactly one of these three subjects?
A train started at 9 AM from a station A with a speed of 72 km/hr. Another train after two hours started from the station B towards A with a speed of 90 km/ph. The two trains are expected to cross each other at 1.30 PM. At 12 noon because of the signals both the trains reduced their speeds by the same quantity and they crossed each other at 4.30 PM. The speed of the train, after 12 noon, that started from the station A, is
The price of a commodity increased by 5% from 2010 to 2011, 8% from 2011 to 2012 and 77% from 2012 to 2013. What is the average price increase (approximate) from 2010 to 2013 ?
Study the given graph and answer the following question.

The number of lecturers recruited in state A in the year 2017 was what percentage of the number of lecturers recruited in state B in the year 2019 ? (Correct to 2 decimal places)
A swimming pool is fitted with 3 pipes A, B, C to fill the pool. A and B together can fill the pool in half the time that is required for C to fill the pool. B takes 20 hours more than the time required for A and 14 hours more than the time required for C to fill the pool. Then the time (in hours) required for all the 3 pipes together to fill the pool is
A and B run in opposite directions from a point P on the circumference of a circle with different but constant speeds. A runs in the clockwise direction. They meet for the first time at distance of 900m in the clockwise direction from P, and for the second time at a distance of 800m in the anticlockwise direction from P, and B is yet to complete one round. The circumference of the circle is:
What is the name of the reaction when acetamide changes into methylamine?
Which type of compounds cannot exhibit geometrical isomerism?
From the options given below, select the option which does not contain the correct isotopes of the elements.
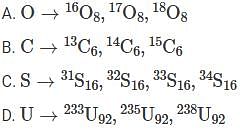
Which of the following is a macronutrient?
The electronegativity of sp2 hybridised atom will be
Which of the following can make difference in optical isomers?
The optimum DP value of cellulose is
Reduction of nitroalkanes yields which compound?
The temperature at which solid and liquid coexist in equilibrium is called
Which of the following is not a secondary pollutant
Which of the following is a not a five membered ring?
Which source of water is free from hardness and surface impurities?
Why are aryl halides less reactive towards nucleophilic substitution reactions as compared to alkyl halides?
If ΔH is the change in enthalpy and ΔE, the change in internal energy accompanying a gaseous reaction, then:
In primary alkyl halides, carbon attached to the halogen atom is further attached to how many carbon atoms?



























