Bihar PGT Chemistry Mock Test - 3 - Bihar PGT/TGT/PRT MCQ
30 Questions MCQ Test Bihar PGT Exam Mock Test Series 2024 - Bihar PGT Chemistry Mock Test - 3
In the following question, an idiomatic expression and its four possible meanings are given. Find out the correct meaning of the idiom.
Q. A house divided against itself cannot stand.
Improve the bracketed part of the sentence with the parts given below.
Q. The cricket teams (have arrive) at the venue yesterday for the match.
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
In the following question, out of the four alternatives, select the word similar in meaning to the word given.
Rumble
The question below consists of a set of labeled sentences. Out of the four options given, select the most logical order of the sentences to form a coherent paragraph.
P: They also went for dinner to an Italian restaurant after the movie.
Q: The bond between them still had been the same though.
R: They had been away for some time and were meeting after a gap.
S: The boys went out for a movie on Friday night.
1 c.c. of 0.1N HCl is added to 99 CC solution of NaCl. The pH of the resulting solution will be
The rate of chemical reaction becomes double for every 10o rise in temperature because of
3 moles of a diatomic gas are heated from 127° C to 727° C at a constant pressure of 1 atm. Entropy change is (log 2.5 = 0 .4)
Ferric hydroxide is a negative sol, which of the following electrolyte will coagulate it most:
When a zinc rod is kept in a copper nitrate solution what happens?
Unleaded paint and petrol were introduced because:
Magnetic moments of the following isoelectronic species (24 electrons) are in order
If equilibrium constant of
CH3COOH + H2O CH3COO- + H3O+
Is 1.8 × 10-5, equilibrium constant for
CH3COOH + OH- CH3COO- + H2O is
In a multi-electron atom, which of the following orbitals described by the three quantum numbers will have the same energy in the absence of magnetic and electric fields?
In the modern periodic table, which period contains 32 elements?
Consider the following reaction,
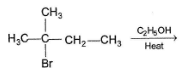
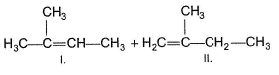
Q.
The correct statement concerning I and II is
Pick out the most reactive alkyl halide for an SN1 reaction.
Which block of the periodic table contains the man made elements?
MnO4- + S2- + H+ → Mn2+ +S + H2O When this equation is balanced, the total number of coefficients on the left hand side are
According to Bohr's theory, the angular momentum of an electron in 5th orbit is - [AIEEE 2006]