Bihar STET Paper 2 Chemistry Mock Test - 1 - Bihar PGT/TGT/PRT MCQ
30 Questions MCQ Test Bihar STET Mock Test Series 2024 - Bihar STET Paper 2 Chemistry Mock Test - 1
Which of the following can be termed as mixed complex?
A vessel of 250 litre was filled with 0.01 mole of Sb2S3 and 0.01 mole of H2 to attain the equilibrium at 440°c as
Sb2S3(s) + 3H2(g) 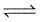 2Sb(s) + 3H2S(g).
2Sb(s) + 3H2S(g).
After equilibrium the H2S formed was analysed by dissolving it in water and treating with excess of Pb2+ to give. 1.195 g of PbS (Molecular weight = 239) precipitate.
What is value of Kc of the reaction at 440°C ?
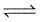 2Sb(s) + 3H2S(g).
2Sb(s) + 3H2S(g).| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which of the following concentration factor is affected by change in temperature ?
[AIEEE-2002]
In which of the following solids, ions of opposite charges are held together by strong electrostatic forces of attraction?
Which one of the following is not a colligative property?
ΔHvap = 30 kJ mol-1 and ΔSvap = 75 J mol-1 K-1. Thus, temperature of vapour at one atmosphere is
[IIT JEE 2004]
For the reaction equilibrium :
N2O4 (g) 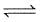 2NO2(g) ; the concentration of N2O4 and NO2 at equilibrium are 4.8 × 10-2 and 1.2 × 10-2 mol/L respectively. The value of Kc for the reaction is :
2NO2(g) ; the concentration of N2O4 and NO2 at equilibrium are 4.8 × 10-2 and 1.2 × 10-2 mol/L respectively. The value of Kc for the reaction is :
In the structure of NaCI given below, ratio rNa+/rcl- is
The lyophobic colloid present in the milk is:
ΔfH° of CS2 = 117.36 kJ mol-1
C(g) = 716.682 kJ mol-1
S(g) = 278.805 kJ mol-1
Q. Thus, bond enthalpy in CS2 is
Only One Option Correct Type
Direction (Q. Nos. 1-8) This section contains 8 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Consider the following reaction,
CH2 = CH2 + Cl2 - H2O → ClCH2 — CH2OH 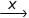 HOCH2CH2OH
HOCH2CH2OH
Q.
The most appropriate reagent X for the above reaction is
In the gas phase water is a bent molecule with a bond angle of
Among the following enthalpies, which is always less than zero?
The electronegativity of following elements increases in the order of?
Only One Option Correct Type
This section contains 7 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct
Q.
The degree of dissociation (α ) o f weak electrolyte AxBy is related to van’t Hoff factor (i) by the expression
[AIEEE 2011]
Which of the following species does not show disproportionation reaction?
Molecular shape of CF4, SF4 and XeF4 are
Direction (Q. Nos. 1-7) This section contains 7 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q.
The hybridisation of N in solid state for N2O5 is
In a 0.2 molal aqueous solution of a weak acid (HX), depression in freezing point is 0.383° Kf is 1.86° mol-1 kg. Assum e molarity equal to molality.
Match the parameters in Column I with their values in Column II and select the answer from the codes given below.
Substances that are strongly attracted by applied magnetic field and can be permanently magnetized are
How many bonding MO are used in the formation of NO?
Direction (Q. Nos. 1-15) This section contains 15 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. By Lewis structure NO has following fact
Only One Option Correct Type
Direction (Q. Nos. 1-8) This section contains 8 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q.
Identify the final major product of the reaction sequence.
Which among the following are the parameters for measuring the validity of a test?
(A) Construct
(B) Stability
(C) Criterion
(D) Content
To make the students learn rhymes more effectively, a primary class teacher should use
The following three aspects of intelligence are dealt with by Sternberg's triarchic theory except:
Primary classes (Classes I to V) will consist of which of the following stages according to NEP, 2020?
A. Preparatory Stage
B. Middle Stage
C. Foundational Stage
A man standing on the terrace of a building watches a car speeding towards him. If at that particular instant the car is 200 m away from the building makes an angle of depression of 60° with the man’s eye and after 8 seconds the angle of depression is 30°, what is the speed of the car?



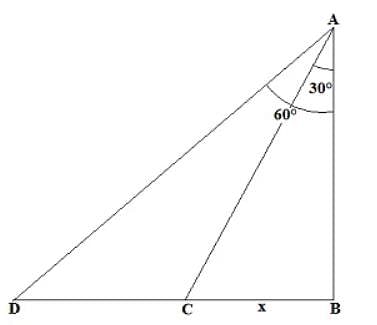
 CD = BD – BC = 200 – 66.67 = 133.33 m
CD = BD – BC = 200 – 66.67 = 133.33 m Speed = 133.33/8 = 16.67 m/s
Speed = 133.33/8 = 16.67 m/s














