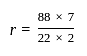NVS PGT Chemistry Mock Test - 8 - NVS TGT/PGT MCQ
30 Questions MCQ Test NVS PGT Mock Test Series 2025 - NVS PGT Chemistry Mock Test - 8
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
In which form of power sharing do we find the arrangement of the system of checks and balances?
From the given alternatives, select the word which CANNOT be formed using the letters of the given word.
FEARLESS
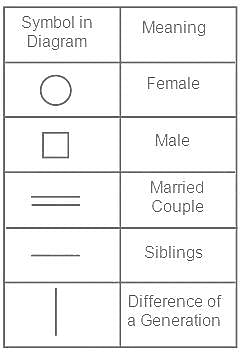
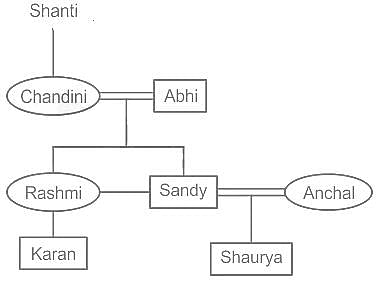
Shanti’s daughter Chandini is married to Abhi. Anchal is married to Sandy, the grandson of Shanti. Abhi's grandson is Karan. Rashmi is the mother of Karan. Shaurya is Anchal's son. How is Shaurya related to Karan?
How do you create a blank line between two lines in a document?
Which of the following would be the characteristic of an effective teacher?
It is said that a teacher is a friend, philosopher and guide because
- a teacher imparts high values to pupils
- a teacher spends a lot of time with the students
- a teacher is a reformer
Choose from the options given below:
Learning disabilities can interfere with learning basic skills such as reading, writing and/or math. These can arise due to all listed reasons except:
The process of evaluation uses several methods because
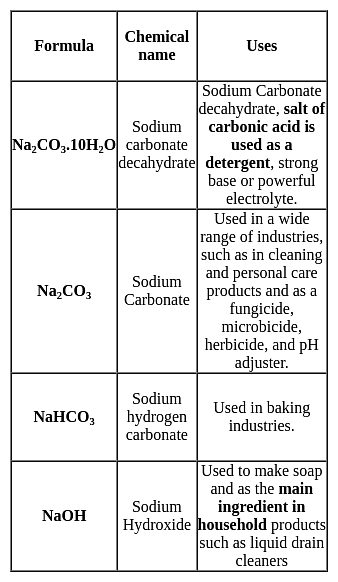
In Dobereiner's Triads, elements were grouped based on their similar chemical properties. Which of the following elements was not part of any known Dobereiner's Triad?
Number of neighbours and next - nearest neighbours of K respectively, are
ΔHvap = 30 kJ mol-1 and ΔSvap = 75 J mol-1 K-1. Thus, temperature of the vapour at 1 atm is
[IIT JEE 2004]
From the following, pick out the potential energy profile for a SN1 reaction.
The alkali metals are low melting. Which of the following alkali metal is expected to melt if the room temperature rises to 30°C?
Type of isomerism exhibited by [Cr(NCS)(NH3)5] [ZnCl4] :
Consider the following reactions,
I. Zn + dil. H2SO4 → ZnSO4 + H2
II. Zn + conc. H2SO4 → ZnSO4+ SO2 + H2O
Oxidising agents in I and II are
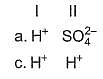
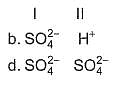
A proton accelerated from rest through a potential difference of 'V' volts has a wavelength λ associated with it. An alpha particle in order to have the same wavelength must be accelerated from rest through a potential difference of
Which of the following strategies will work for mentally retarded children?
Which of the following is NOT a form of non-discursive communication?
Which of the following is proposed in National Education Policy 2020?
(i) Learning how to learn
(ii) Increasing course content
(iii) 360-degree holistic progress report card
(iv) Standardized curriculum, pedagogy, and assessment
Choose the correct option.
The following sentence has been broken into four parts with an error in one part. Identify that part and mark it as your answer. If there are no errors in any of the given parts, mark option 4 or ‘No error’ as your answer.
Q. The student, whom you (1)/ expected to win the prize, (2)/ had lost miserably.(3)/ No error (4).
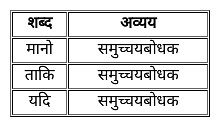
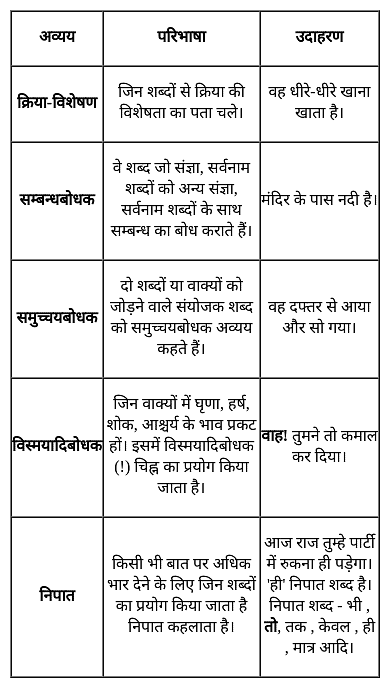
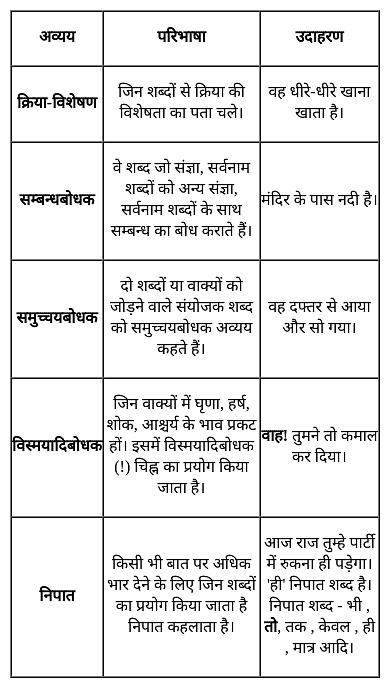
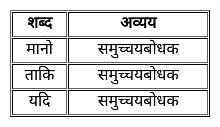
निम्न में से कौन-सा शब्द निपात का उदाहरण नहीं है?
|
30 tests
|