Case Based Questions Test: Thermodynamics - NEET MCQ
10 Questions MCQ Test Topic-wise MCQ Tests for NEET - Case Based Questions Test: Thermodynamics
Attempt All sub parts from each question.
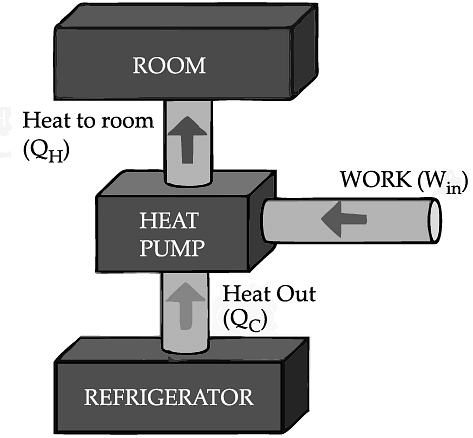
If we turn on our electric oven, and it will warm up the room. Keeping the oven door open, the room gets warm even faster. This is obvious since the purpose of an oven is to make things hot. But the opposite is not true of a refrigerator. Running the refrigerator makes the room warmer. If the door is kept open, the room gets warm up even faster. The first rush of cold air may cool things down a little, but in the long run, the room will get warmer. To see why, we need to think of heat as energy and cold as a lack of energy. The stove produces heat, but the refrigerator can't actually produce cold. All the refrigerator does is move heat, or energy, from one place to another. As the food inside the refrigerator loses its heat or, in other words, gets colder that heat warms up the room A refrigerator is an open system that dispels heat from a closed space to a warmer area, usually in the room. By dispelling the heat from this area, it decreases the temperature inside, allowing food and other items to remain cool. Refrigerators appear to violate the second law of thermodynamics. But actually they do not. Physicists call this kind of system a "heat pump." According to the Second Law of Thermodynamics, heat always flows from hot to cold, and not in the other way around. A refrigerator causes heat to flow from cold to hot by inputting work, which cools the space inside it.
It does this by following the steps below:
• Work is inputted (Win) which compresses a coolant, increases its temperature above the room temperature.
• Heat flows from coolant into the room (QH) reducing the temperature of the coolant.
• The coolant expands and it cools down below the temperature inside the refrigerator.
• Heat flows from the refrigerator to the coolant (QC) decreasing the temperature inside.

So, the heat pump in refrigerator needs energy to run. So while it is busy in moving energy out of the refrigerator and into the room, it also draws more energy in the form of electricity. Since some of that energy is released as heat, the room gets warmer.
For refrigerators, a manufacturer would want to make the area colder while doing as little work as possible. By doing little work to cool the appliance, the refrigerator can stay at the desired temperature while using less electricity. The number that describes this idea is known as the coefficient of performance, K, which is essentially a measure of efficiency.
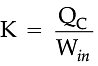
The higher this value is, the better the refrigerator, because it means that less work is being done to cool down the refrigerator.
Q. Which of the following statement is true?

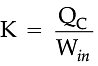
Attempt All sub parts from each question.
If we turn on our electric oven, and it will warm up the room. Keeping the oven door open, the room gets warm even faster. This is obvious since the purpose of an oven is to make things hot. But the opposite is not true of a refrigerator. Running the refrigerator makes the room warmer. If the door is kept open, the room gets warm up even faster. The first rush of cold air may cool things down a little, but in the long run, the room will get warmer. To see why, we need to think of heat as energy and cold as a lack of energy. The stove produces heat, but the refrigerator can't actually produce cold. All the refrigerator does is move heat, or energy, from one place to another. As the food inside the refrigerator loses its heat or, in other words, gets colder that heat warms up the room A refrigerator is an open system that dispels heat from a closed space to a warmer area, usually in the room. By dispelling the heat from this area, it decreases the temperature inside, allowing food and other items to remain cool. Refrigerators appear to violate the second law of thermodynamics. But actually they do not. Physicists call this kind of system a "heat pump." According to the Second Law of Thermodynamics, heat always flows from hot to cold, and not in the other way around. A refrigerator causes heat to flow from cold to hot by inputting work, which cools the space inside it.
It does this by following the steps below:
• Work is inputted (Win) which compresses a coolant, increases its temperature above the room temperature.
• Heat flows from coolant into the room (QH) reducing the temperature of the coolant.
• The coolant expands and it cools down below the temperature inside the refrigerator.
• Heat flows from the refrigerator to the coolant (QC) decreasing the temperature inside.
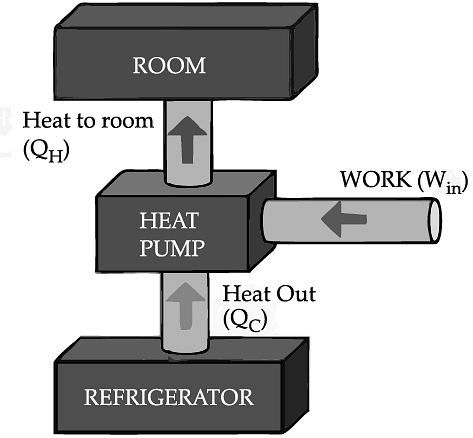
So, the heat pump in refrigerator needs energy to run. So while it is busy in moving energy out of the refrigerator and into the room, it also draws more energy in the form of electricity. Since some of that energy is released as heat, the room gets warmer.
For refrigerators, a manufacturer would want to make the area colder while doing as little work as possible. By doing little work to cool the appliance, the refrigerator can stay at the desired temperature while using less electricity. The number that describes this idea is known as the coefficient of performance, K, which is essentially a measure of efficiency.

The higher this value is, the better the refrigerator, because it means that less work is being done to cool down the refrigerator.
Q. Keeping the door of a refrigeration open the room temperature


| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Attempt All sub parts from each question.
If we turn on our electric oven, and it will warm up the room. Keeping the oven door open, the room gets warm even faster. This is obvious since the purpose of an oven is to make things hot. But the opposite is not true of a refrigerator. Running the refrigerator makes the room warmer. If the door is kept open, the room gets warm up even faster. The first rush of cold air may cool things down a little, but in the long run, the room will get warmer. To see why, we need to think of heat as energy and cold as a lack of energy. The stove produces heat, but the refrigerator can't actually produce cold. All the refrigerator does is move heat, or energy, from one place to another. As the food inside the refrigerator loses its heat or, in other words, gets colder that heat warms up the room A refrigerator is an open system that dispels heat from a closed space to a warmer area, usually in the room. By dispelling the heat from this area, it decreases the temperature inside, allowing food and other items to remain cool. Refrigerators appear to violate the second law of thermodynamics. But actually they do not. Physicists call this kind of system a "heat pump." According to the Second Law of Thermodynamics, heat always flows from hot to cold, and not in the other way around. A refrigerator causes heat to flow from cold to hot by inputting work, which cools the space inside it.
It does this by following the steps below:
• Work is inputted (Win) which compresses a coolant, increases its temperature above the room temperature.
• Heat flows from coolant into the room (QH) reducing the temperature of the coolant.
• The coolant expands and it cools down below the temperature inside the refrigerator.
• Heat flows from the refrigerator to the coolant (QC) decreasing the temperature inside.
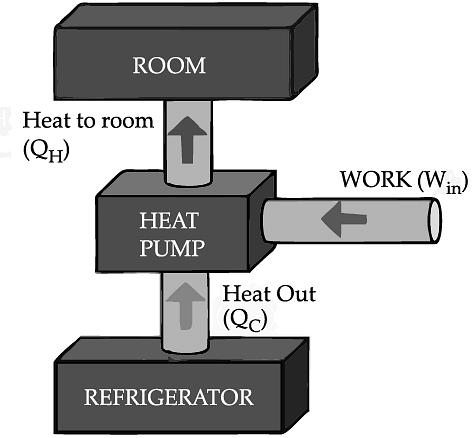
So, the heat pump in refrigerator needs energy to run. So while it is busy in moving energy out of the refrigerator and into the room, it also draws more energy in the form of electricity. Since some of that energy is released as heat, the room gets warmer.
For refrigerators, a manufacturer would want to make the area colder while doing as little work as possible. By doing little work to cool the appliance, the refrigerator can stay at the desired temperature while using less electricity. The number that describes this idea is known as the coefficient of performance, K, which is essentially a measure of efficiency.
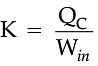
The higher this value is, the better the refrigerator, because it means that less work is being done to cool down the refrigerator.
Q. 2nd law of thermodynamics states:

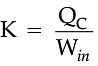
Attempt All sub parts from each question.
If we turn on our electric oven, and it will warm up the room. Keeping the oven door open, the room gets warm even faster. This is obvious since the purpose of an oven is to make things hot. But the opposite is not true of a refrigerator. Running the refrigerator makes the room warmer. If the door is kept open, the room gets warm up even faster. The first rush of cold air may cool things down a little, but in the long run, the room will get warmer. To see why, we need to think of heat as energy and cold as a lack of energy. The stove produces heat, but the refrigerator can't actually produce cold. All the refrigerator does is move heat, or energy, from one place to another. As the food inside the refrigerator loses its heat or, in other words, gets colder that heat warms up the room A refrigerator is an open system that dispels heat from a closed space to a warmer area, usually in the room. By dispelling the heat from this area, it decreases the temperature inside, allowing food and other items to remain cool. Refrigerators appear to violate the second law of thermodynamics. But actually they do not. Physicists call this kind of system a "heat pump." According to the Second Law of Thermodynamics, heat always flows from hot to cold, and not in the other way around. A refrigerator causes heat to flow from cold to hot by inputting work, which cools the space inside it.
It does this by following the steps below:
• Work is inputted (Win) which compresses a coolant, increases its temperature above the room temperature.
• Heat flows from coolant into the room (QH) reducing the temperature of the coolant.
• The coolant expands and it cools down below the temperature inside the refrigerator.
• Heat flows from the refrigerator to the coolant (QC) decreasing the temperature inside.

So, the heat pump in refrigerator needs energy to run. So while it is busy in moving energy out of the refrigerator and into the room, it also draws more energy in the form of electricity. Since some of that energy is released as heat, the room gets warmer.
For refrigerators, a manufacturer would want to make the area colder while doing as little work as possible. By doing little work to cool the appliance, the refrigerator can stay at the desired temperature while using less electricity. The number that describes this idea is known as the coefficient of performance, K, which is essentially a measure of efficiency.

The higher this value is, the better the refrigerator, because it means that less work is being done to cool down the refrigerator.
Q. In refrigerator, work is inputted which
Attempt All sub parts from each question.
If we turn on our electric oven, and it will warm up the room. Keeping the oven door open, the room gets warm even faster. This is obvious since the purpose of an oven is to make things hot. But the opposite is not true of a refrigerator. Running the refrigerator makes the room warmer. If the door is kept open, the room gets warm up even faster. The first rush of cold air may cool things down a little, but in the long run, the room will get warmer. To see why, we need to think of heat as energy and cold as a lack of energy. The stove produces heat, but the refrigerator can't actually produce cold. All the refrigerator does is move heat, or energy, from one place to another. As the food inside the refrigerator loses its heat or, in other words, gets colder that heat warms up the room A refrigerator is an open system that dispels heat from a closed space to a warmer area, usually in the room. By dispelling the heat from this area, it decreases the temperature inside, allowing food and other items to remain cool. Refrigerators appear to violate the second law of thermodynamics. But actually they do not. Physicists call this kind of system a "heat pump." According to the Second Law of Thermodynamics, heat always flows from hot to cold, and not in the other way around. A refrigerator causes heat to flow from cold to hot by inputting work, which cools the space inside it.
It does this by following the steps below:
• Work is inputted (Win) which compresses a coolant, increases its temperature above the room temperature.
• Heat flows from coolant into the room (QH) reducing the temperature of the coolant.
• The coolant expands and it cools down below the temperature inside the refrigerator.
• Heat flows from the refrigerator to the coolant (QC) decreasing the temperature inside.
So, the heat pump in refrigerator needs energy to run. So while it is busy in moving energy out of the refrigerator and into the room, it also draws more energy in the form of electricity. Since some of that energy is released as heat, the room gets warmer.
For refrigerators, a manufacturer would want to make the area colder while doing as little work as possible. By doing little work to cool the appliance, the refrigerator can stay at the desired temperature while using less electricity. The number that describes this idea is known as the coefficient of performance, K, which is essentially a measure of efficiency.

The higher this value is, the better the refrigerator, because it means that less work is being done to cool down the refrigerator.
Q. Performance of refrigerator is based on ............... law of thermodynamics.
Importance of high specific heat capacity of water for life Specific heat capacity of a substance is the amount of heat required to raise the temperature of that substance by 1 K. It is expressed in the units J/ (kg K). A high specific heat of a substance means that a large amount of heat is required to raise the temperature of the substance. Water has the highest known specific heat capacity. Its specific heat capacity is 4.186 K J/ (kg K) i.e. to raise the temperature of 1 kg of water by 1 Kelvin it requires 4.186 KJ of heat. For comparison sake, Copper requires only 385 Joules of heat to raise 1 kilogram of copper by 1 Kelvin. It also interesting to know that the specific heat capacities in two other phases of water (i.e. ice and water vapour) are less than this. High specific heat of water is mainly due to the presence of a large number of hydrogen bonds between molecules of water. On a beach on a sunny day, it is noticed that the sand is often quite hot to walk on, but the water is always cool, even in the shallows. This is because sand has a lower specific heat capacity than that of water. Sand takes less energy to raise the temperature by one degree. Because water has a high specific heat capacity, it requires more energy to raise the temperature by one degree. Since the sun puts same rate of energy on water and sand, which heats up sand more quickly and water more slowly. Water covers around 70% of the Earth's surface and its high specific heat plays a very important role to sustain life in the earth. It is able to absorb a lot of heat without a significant rise in the temperature. When temperatures decrease, the heat which is stored is released, restraining a rapid drop in temperature. The combined effect is the buffering of heat. A relatively constant temperature without sudden rise and drop is essential to sustain life. Hence water is important for life.
Q. Specific heat capacity of water is
Importance of high specific heat capacity of water for life Specific heat capacity of a substance is the amount of heat required to raise the temperature of that substance by 1 K. It is expressed in the units J/ (kg K). A high specific heat of a substance means that a large amount of heat is required to raise the temperature of the substance. Water has the highest known specific heat capacity. Its specific heat capacity is 4.186 K J/ (kg K) i.e. to raise the temperature of 1 kg of water by 1 Kelvin it requires 4.186 KJ of heat. For comparison sake, Copper requires only 385 Joules of heat to raise 1 kilogram of copper by 1 Kelvin. It also interesting to know that the specific heat capacities in two other phases of water (i.e. ice and water vapour) are less than this. High specific heat of water is mainly due to the presence of a large number of hydrogen bonds between molecules of water. On a beach on a sunny day, it is noticed that the sand is often quite hot to walk on, but the water is always cool, even in the shallows. This is because sand has a lower specific heat capacity than that of water. Sand takes less energy to raise the temperature by one degree. Because water has a high specific heat capacity, it requires more energy to raise the temperature by one degree. Since the sun puts same rate of energy on water and sand, which heats up sand more quickly and water more slowly. Water covers around 70% of the Earth's surface and its high specific heat plays a very important role to sustain life in the earth. It is able to absorb a lot of heat without a significant rise in the temperature. When temperatures decrease, the heat which is stored is released, restraining a rapid drop in temperature. The combined effect is the buffering of heat. A relatively constant temperature without sudden rise and drop is essential to sustain life. Hence water is important for life.
Q. To raise the temperature of 1 kg of water and 1 kg of copper by 1 Kelvin, 4.186 KJ and 385 Joule of heat are required respectively. Which one will get heated up faster if exposed to sun?
Importance of high specific heat capacity of water for life Specific heat capacity of a substance is the amount of heat required to raise the temperature of that substance by 1 K. It is expressed in the units J/ (kg K). A high specific heat of a substance means that a large amount of heat is required to raise the temperature of the substance. Water has the highest known specific heat capacity. Its specific heat capacity is 4.186 K J/ (kg K) i.e. to raise the temperature of 1 kg of water by 1 Kelvin it requires 4.186 KJ of heat. For comparison sake, Copper requires only 385 Joules of heat to raise 1 kilogram of copper by 1 Kelvin. It also interesting to know that the specific heat capacities in two other phases of water (i.e. ice and water vapour) are less than this. High specific heat of water is mainly due to the presence of a large number of hydrogen bonds between molecules of water. On a beach on a sunny day, it is noticed that the sand is often quite hot to walk on, but the water is always cool, even in the shallows. This is because sand has a lower specific heat capacity than that of water. Sand takes less energy to raise the temperature by one degree. Because water has a high specific heat capacity, it requires more energy to raise the temperature by one degree. Since the sun puts same rate of energy on water and sand, which heats up sand more quickly and water more slowly. Water covers around 70% of the Earth's surface and its high specific heat plays a very important role to sustain life in the earth. It is able to absorb a lot of heat without a significant rise in the temperature. When temperatures decrease, the heat which is stored is released, restraining a rapid drop in temperature. The combined effect is the buffering of heat. A relatively constant temperature without sudden rise and drop is essential to sustain life. Hence water is important for life.
Q. Specific heat capacity of ice is ............... than and specific heat capacity of water vapour is ............... than that of water.
Importance of high specific heat capacity of water for life Specific heat capacity of a substance is the amount of heat required to raise the temperature of that substance by 1 K. It is expressed in the units J/ (kg K). A high specific heat of a substance means that a large amount of heat is required to raise the temperature of the substance. Water has the highest known specific heat capacity. Its specific heat capacity is 4.186 K J/ (kg K) i.e. to raise the temperature of 1 kg of water by 1 Kelvin it requires 4.186 KJ of heat. For comparison sake, Copper requires only 385 Joules of heat to raise 1 kilogram of copper by 1 Kelvin. It also interesting to know that the specific heat capacities in two other phases of water (i.e. ice and water vapour) are less than this. High specific heat of water is mainly due to the presence of a large number of hydrogen bonds between molecules of water. On a beach on a sunny day, it is noticed that the sand is often quite hot to walk on, but the water is always cool, even in the shallows. This is because sand has a lower specific heat capacity than that of water. Sand takes less energy to raise the temperature by one degree. Because water has a high specific heat capacity, it requires more energy to raise the temperature by one degree. Since the sun puts same rate of energy on water and sand, which heats up sand more quickly and water more slowly. Water covers around 70% of the Earth's surface and its high specific heat plays a very important role to sustain life in the earth. It is able to absorb a lot of heat without a significant rise in the temperature. When temperatures decrease, the heat which is stored is released, restraining a rapid drop in temperature. The combined effect is the buffering of heat. A relatively constant temperature without sudden rise and drop is essential to sustain life. Hence water is important for life.
Q. Which statement is false?
Importance of high specific heat capacity of water for life Specific heat capacity of a substance is the amount of heat required to raise the temperature of that substance by 1 K. It is expressed in the units J/ (kg K). A high specific heat of a substance means that a large amount of heat is required to raise the temperature of the substance. Water has the highest known specific heat capacity. Its specific heat capacity is 4.186 K J/ (kg K) i.e. to raise the temperature of 1 kg of water by 1 Kelvin it requires 4.186 KJ of heat. For comparison sake, Copper requires only 385 Joules of heat to raise 1 kilogram of copper by 1 Kelvin. It also interesting to know that the specific heat capacities in two other phases of water (i.e. ice and water vapour) are less than this. High specific heat of water is mainly due to the presence of a large number of hydrogen bonds between molecules of water. On a beach on a sunny day, it is noticed that the sand is often quite hot to walk on, but the water is always cool, even in the shallows. This is because sand has a lower specific heat capacity than that of water. Sand takes less energy to raise the temperature by one degree. Because water has a high specific heat capacity, it requires more energy to raise the temperature by one degree. Since the sun puts same rate of energy on water and sand, which heats up sand more quickly and water more slowly. Water covers around 70% of the Earth's surface and its high specific heat plays a very important role to sustain life in the earth. It is able to absorb a lot of heat without a significant rise in the temperature. When temperatures decrease, the heat which is stored is released, restraining a rapid drop in temperature. The combined effect is the buffering of heat. A relatively constant temperature without sudden rise and drop is essential to sustain life. Hence water is important for life.
Q. On a beach on a sunny day, it is noticed that the sand is often quite hot , but the water is always cool. This is due to
|
9 docs|1272 tests
|


















