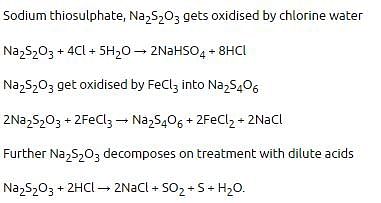Test: p-Block Elements - 1 - NEET MCQ
25 Questions MCQ Test Chemistry Class 11 - Test: p-Block Elements - 1
The elements of group 14 are slightly more electronegative than group 13 elements because of
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
In which of the following compounds, nitrogen exhibits highest oxidation state?
Among the following which is the strongest oxidizing agent?
The compounds formed by highly reactive non-metals with highly reactive metals are generally
A compound X, of boron reacts with NH3 on heating to give another compound. Y which is called inorganic benzene. The compound X can be prepared by treating BF3 with Lithium aluminium hydride. The compounds X and Y are represented by the formulas.
Three reactions involving are given below
B.
C.
In which of the above does act as an acid?
When Cl2 gas reacts with hot and concentrated sodium hydroxide solution, the oxidation number of chlorine changes from
As we move from B to Al in the p-block elements the sum of the first three ionisation enthalpies
The tendency of BF3, BCl3 and BBr3 to behave as Lewis acid decreases in the sequence:
Boron forms only covalent compounds and not +3 ions because
The element having the noble gas plus 14 f- electrons plus 10 d-electron cores
In trivalent state, for example, the trichlorides, being covalent are hydrolysed in water form
Which of the following statements regarding ozone is not correct?
Aqueous solution of Na2S2O3 on reaction with Cl2 gives
Aqueous solution of Na2S2O3 on reaction with Cl2 gives
One of the following p-block elements has unusually low melting point
In the manufacture of sulphuric acid by contact process Tyndall box is used to
|
129 videos|244 docs|88 tests
|